- 玉米蛇宠物饲养询证指南
- 说明
- 本指南建设原则
- 入手前需要什么
- 基本物种信息
- 解剖与生理
- 动物福利总论
- 蛇的行为学
- 爬行动物的认知能力
- 丰容
- 玉米蛇的自然生境
- 玉米蛇饲养的要素
- 空间需求
- 栖地布置总则
- 光照与加热
- 光照的测试
- 湿度
- 垫材
- 攀爬、遮挡与躲避
- 水
- 食物
- 共栖关系
- 蜕皮
- 特殊状态:冬眠和繁殖
- 互动与行为训练
- 运输
- 摄像头
- 伏地魔栖地
- 应急预案
- 蛇的医疗
- 信息来源
- 其他问题
- 词汇表
- 其他物种
【】【那个冯柚子有一个问题宝宝系列】【】
https://reptifiles.com/corn-snake-care-guide/sick-corn-snake-diseases-health/
玉米蛇的常见疾病:【】【Marie Kubiak - Handbook of Exotic Pet Medicine (2020, John Wiley & Sons, Incorporated) - libgen.li】【】
16.4 Common Medical and Surgical Conditions
16.4.2 Dysecdysis
The outer keratinised portion of skin is shed in a single piece every three to four months in adults, and this process is termed ecdysis. Growing juveniles shed more frequently at every three to eight weeks (Penning 2012). Low temperatures, ill health, and malnutrition may extend the shedding cycle, whereas ectoparasitism, dermatitis, or endocrine disease may increase shedding frequency (Harkewicz 2002). Disruption to the shedding process, including incomplete or protracted ecdysis, is termed dysecdysis. Inappropriate humidity, dehydration, dermal injury, inflammation or scarring, and debilitation are common factors in dysecdysis. Retained areas of skin may cause irritation or act as a nidus for infection. If the distal tail is affected then progressive contraction of unshed skin can cause avascular necrosis of the tail tip (Harkewicz 2002). Soaking the snake in tepid water for 20–30 minutes can aid in atraumatic removal of retained skin using a damp towel or forceps. Shed skin should not be forcefully removed as this may damage the underlying skin.
In snakes, the eyelids are replaced by a clear skin layer, termed the spectacle (van Doorn and Sivak 2013). The spectacles may be retained in dysecdysis, giving a dull appearance to the eye. This can be seen with generalised dysecdysis, or with localised irritation, such as periocular snake mite infestation. Applying hypromellose drops repeatedly over a 30 minute period may aid in separating the old and new spectacle at the periphery, allowing gentle removal with a cotton bud or forceps. Forceful removal can damage the new spectacle, with exposure of the cornea and potential for permanent eye damage. If the spectacle is not readily removed then correcting causative factors and leaving it to be cast at the next shed is recommended.
Hyperthyroidism is described as a cause of increased frequency of ecdysis in corn snakes, due to primary hyperthyroidism, or hyperthyroidism secondary to pituitary pathology (Whiteside and Gamer 2001; Harkewicz 2002). However, convincing evidence of clinical hyperthyroidism is lacking and reports suggests that hyperthyroidism should reduce rather than increase the frequency of ecdysis (Chiu and Lynn 1970; Chiu et al. 1983; Hunt 2015). Serum thyroxine levels can be assessed in cases of atypical ecdysis frequency, the normal range for free T4 in this species is reported to be 0.45–6.06 nmol/l (Greenacre et al. 2001).
16.4.3 Dermatitis
Dermatitis may present as increased shedding frequency, localised skin retention, alteration of pigmentation, swelling, abscessation, exudative ulceration, or sloughing of tissue (Hoppmann and Barron 2007). Thermal, chemical, bacterial, and fungal causes are well recognised.
Thermal burns are a major differential for dermal injury in pet snakes (Hoppmann and Barron 2007). It has been hypothesised that snakes process thermal stimuli differently to other animals, with a reduced response to noxious thermal stimuli seen (Sladky et al. 2008). This may explain the failure of snakes to move away from malfunctioning or inappropriate heat sources, resulting in contact burns that can be extensive. No external damage may be seen immediately after thermal insult and most snakes only present days later once sloughing of damaged tissues commences. Open wounds may result over a large area of the body surface with potential for significant loss of fluid and protein in exudates, and opportunistic infection. All cases require analgesia and replacing substrate with paper towel or a bare vivarium floor temporarily will aid in keeping traumatised areas clean.
First degree burns are superficial, affecting only the epidermis with erythema and blistering often resulting (Mader 2002). Topical treatment such as silver sulphasalazine or zinc oxide will aid healing, with resolution of lesions in a four- to six-week period.
Second degree burns are associated with necrosis of the epidermis and damage to the deeper dermis. Swelling, blistering, discoloration of skin, and exudative lesions are seen. Systemic antibiotic therapy and topical treatments are necessary for these cases (Harkewicz 2002) but scarring can result. Adhesive sterile dressings can be placed over injuries to prolong action of topical agents and protect the wound (Mader 2002). Maintaining snakes at the top end of their temperature gradient will accelerate healing (Smith et al. 1988). Fluid therapy requirements will depend on the area of the burn and the fluid losses sustained.
Third degree burns have full thickness skin damage with sloughing of skin across the lesion. Fluid therapy is necessary as losses can be significant. Daily bandage changes, systemic and topical antibiotics, and multimodal analgesia are necessary for management of these cases in the first days to weeks of treatment, with ongoing medical therapy and less frequent bandage changes required for the four to six month healing period (Mader 2002). Significant scarring will result and may interfere with ecdysis.
Bacterial or fungal infections are rarely primary concerns, but follow insult to skin integrity such as a burn or bite from prey, or chronic husbandry deficiency such as excessive or insufficient humidity, low environmental temperatures, obesity, unsanitary conditions, or malnutrition (Harkewicz 2002; Hoppmann and Barron 2007). Where substrate is consistently wet or heavily contaminated, the ventrum is most commonly affected and may appear erythematous and develop vesicles or ulceration (Hoppmann and Barron 2007). The underlying cause should be corrected. Skin lesions should be cultured to guide therapy, and antimicrobials, analgesia, topical disinfectants, and debridement and subsequent protection of affected areas using bandaging considered based on severity of lesions. Fungal lesions may require biopsy for confirmation of diagnosis (Hoppmann and Barron 2007).
Primary fungal pathogens are uncommon in captive animals, but Ophidiomyces ophiodiicola (formerly Chrysosporium ophiodiicola) is an emerging disease in wild and captive snake populations and has been reported in colubrid snakes (Allender et al. 2015). Infection in most snakes affects the head and ventral scales with caseation and crusts but the one clinical case reported in a corn snake presented with a subcutaneous nodule (Sigler et al. 2013). O. ophiodiicola has also been demonstrated to cause disease in experimentally infected corn snakes (Lorch et al. 2015). Infected animals in this study developed oedema, crusting, and hyperpigmentation of scales, increased ecdysis frequency and dysecdysis but appeared able to clear the fungal infection following ecdysis (Lorch et al. 2015). For clinical cases, biopsy for fungal culture and histology is recommended, with treatment comprising systemic and topical antifungals. O. ophiodiicola appears poorly responsive to itraconazole and ketoconazole therapy, and voriconazole has been associated with death of snakes at 5 mg/kg (Rajeev et al. 2009; Sigler et al. 2013; Lindemann et al. 2017). Terbinafine has been demonstrated to be effective in vitro, and reaches expected therapeutic levels in c ottonmouths (Agkistrodon piscivorous) with nebulisation or placement of a subcutaneous implant (Kane et al. 2017). Based on the study findings, 30 minutes n ebulisation daily using a 2 mg/ml terbinafine solution was r ecommended as a potential treatment. Oral and topical terbinafine formulations are commercially available but no dosing schedules have been determined. Exposure of spores for 2 minutes to 3% bleach, or for 10 minutes to 70% ethanol or quaternary ammonium products have been shown to be effective for disinfection, but c hlorhexidine and propiconazole were ineffective (Rzadkowska et al. 2016).
16.4.4 Respiratory Disease
In snakes, the right lung is a simple sac, lined with respiratory epithelium cranially and avascular non-respiratory epithelium caudally, resulting in a relatively small surface area for gas exchange (Schumacher 2003). The left lung is vestigial in colubrids. Clinical respiratory disease is common in pet snakes but in the author’s experience, respiratory disease is less common in colubrid snakes than larger, sedentary boids. Clinical signs include nasal discharge, audible respiratory noise, presence of secretions in the oral cavity, stomatitis, dyspnoea, open mouth breathing, elevation of the head and neck, and cyanosis (Schumacher 2003). Disease is often advanced by the time of overt symptoms and presentation. Radiography is of limited value for mild–moderate disease but may show abscess formation, fluid accumulation, or increased radiodensity of the lung in advanced cases. Computed tomography is of higher sensitivity for lesions and is preferred for diagnosing and monitoring respiratory disease but is not as readily available. Ultrasonography may identify increases in fluid or soft tissue and facilitate collection of aspirate samples for culture or cytology (Schumacher 2003). Endoscopy of the trachea is limited by patient size, but swabs can often be collected for culture and PCR diagnostics for upper respiratory tract diseases. Tracheal and lung wash samples are advisable to determine specific pathogen presence in lower respiratory tract disease (Murray 2005). Percutaneous surgical endoscopic access to the lung can be utilised for sample collection or topical therapy of focal lesions.
Bacteria, particularly gram-negative organisms, are commonly isolated from respiratory cases and may be primary pathogens, opportunistic invaders, or co-infections.
Viral respiratory pathogens reported in corn snakes include ophidian paramyxovirus, reovirus, and adenovirus (Abbas et al. 2011). These viruses may be present in clinical cases, asymptomatic animals, or as part of mixed infections (Abbas et al. 2011), so a positive result should be interpreted in accordance with the animal’s clinical status. For paramyxovirus, demonstration of a rising titre is recommended to confirm active infection (Jacobson and Origgi 2002). It is unknown if snakes with static antibody levels have cleared infection or remain permanently infected, though a small proportion of corn snakes experimentally infected with paramyxovirus appeared able to clear infection (Pees et al. 2016). Supportive care may allow recovery in less severe cases. These viruses are highly infectious and can spread within and between collections readily, emphasising the importance of biosecurity, quarantine, and targeted screening of new animals.
Non-infectious respiratory disease is uncommon but may include penetrating injuries, neoplasia, or exposure to irritant gases or aerosols.
Treatment of respiratory disease involves targeted therapy for the primary cause where possible, husbandry improvements, and supportive measures. The lack of a diaphragm, reliance on skeletal muscles for air movement, and lack of a developed mucocilary system means that respiratory secretion clearance is poor in snakes. Fluid therapy, nebulisation, encouraging activity, coupage and drainage of secretions by elevating the mid body above the head may aid clearance of debris in lower respiratory tract disease. Maintaining animals at the top end of their thermal range will aid immune function and maintaining a high humidity level may decrease secretion viscosity, enhancing clearance.
16.4.5 Cardiac Disease
A detailed description of cardiac anatomy and physiology in snakes is beyond the scope of this chapter but major differences will be mentioned. Cranially there are two atria, and caudally a single ventricle, subdivided by a muscular ridge – the vertical septum. The thin-walled sinus venosus, is located dorsal to the right atrium and receives blood from the systemic circulation, contracting prior to atrial contraction to aid filling of the right atrium (Farrell et al. 1998; Jensen et al. 2014). During respiration, the ventricle is functionally divided by the vertical septum to function as a bi-chambered heart, in an equivalent way to mammals. When apnoeic, parasympathetic tone increases resulting in bradycardia and increased pulmonary resistance. Under these conditions blood from the right side of the heart is shunted across the ventricle and a significant proportion of the blood re-enters the systemic circulation, bypassing the pulmonary circulation (Bogan 2017). This reduces cardiac activity when oxygen is unavailable, but maintains perfusion to the systemic circulation.
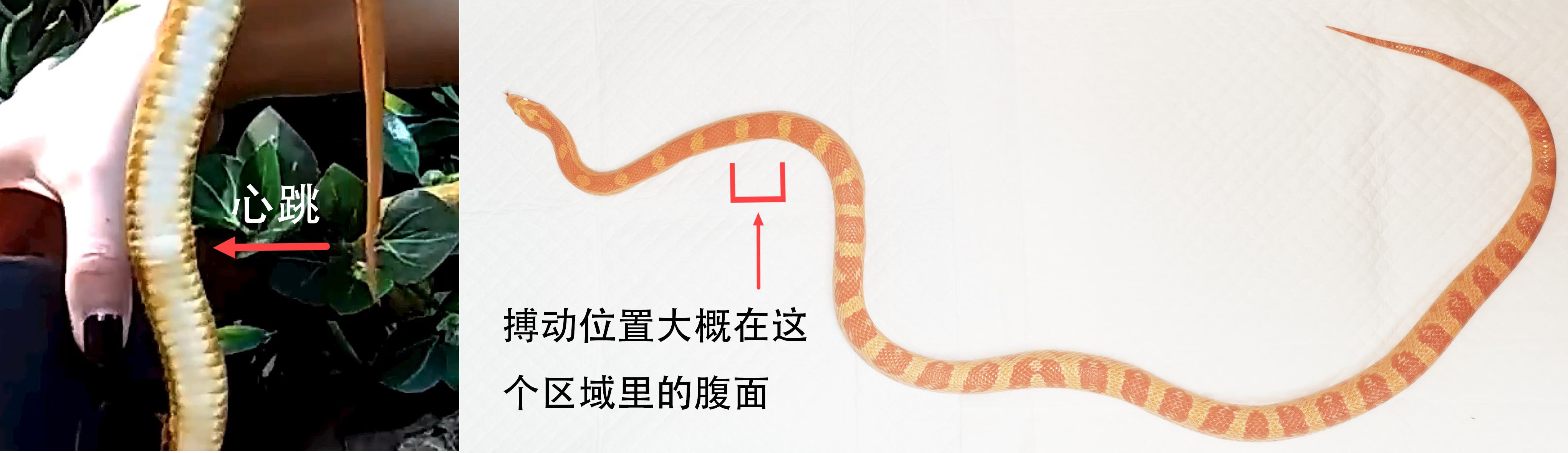
Cardiac disease may present with regurgitation, anorexia, lethargy, weight loss, peripheral oedema, cyanosis, open mouth breathing, or a discrete swelling (Figure 16.8) (Kik and Mitchell 2005; Beaufrère et al. 2016). Assessment should take place with the snake at an appropriate temperature as cardiac function can alter with hyper- or hypothermia. The cardiac position can be localised on examination by placing the snake in dorsal recumbency and visualising a pulsatile region approximately 20–23% along the length of the body of the snake (Divers 2008). Auscultation with a stethoscope is unrewarding, but Doppler ultrasonic probes can be useful in assessing for alterations in heart sounds (Kik and Mitchell 2005; Mitchell 2009). In another colubrid, the yellow rat snake (Elaphe obsoleta quadrivitatta), creatine kinase rises with myocardial damage and may be useful in supporting a diagnosis of cardiomyopathy (Ramsay and Dotson 1995). For electrocardiography (ECG), lead II is most frequently used. The negative lead is placed one to two heart lengths cranial to the heart on the right, the positive lead placed on the left, approximately 60–75% along the body and the neutral lead placed on the right, opposite the positive lead (Kik and Mitchell 2005; Bogan 2017). The trace has P and T waves and a QRS complex, similar to that seen in mammals but amplitude may be lower, hindering interpretation (Kik and Mitchell 2005). An additional ‘SV’ wave may been seen prior to the P wave, reflecting depolarisation of the sinus venosus (Martinez-Silvestre et al. 2003). ECG interpretation is not yet well described in snakes and caution should be exercised when making a diagnosis based on an ECG alone.
Figure 16.8 Pronounced pulsatile swelling in the cranial coelom indicative of cardiomegaly. Cardiac disease appears common in colubrid snakes.
Radiography, particularly the laterolateral view of the cranial-mid body, may support cardiac disease, with c ardiomegaly, hepatomegaly, pulmonary oedema, vascular mineralisation, or ascites potential findings (Mitchell 2009). Echocardiography may be hampered by poor acoustic coupling but soaking the snake in warm water prior to scanning and using large volumes of coupling gel will aid image quality. A ventral approach is advised, directly over the heart (Schilliger et al. 2006). Potential pathology that can be detected includes pericardial effusion, alterations in valve function or anatomy, parasite presence, neoplasia, or mineralisation of tissues (Bogan 2017). Chamber dilation or thickening of the myocardium may be suspected on echocardiography, but objective assessment is not possible due to a lack of normal values for this species. Cardiomegaly has been reported in this species but prevalence is undetermined (Kik and Mitchell 2005). The author has seen a disproportionately high prevalence of dilated cardiomyopathy in corn and rat snakes, but no evident primary cause has been identified. Treatment for cardiac disease is extrapolated from therapy in domestic mammals though dosing regimens are not validated.
16.4.6 Stomatitis
Oral examination is readily achieved in a restrained snake by gently passing a flat card or blunt probe into the mouth from the diastema at the rostral tip to open the mouth. There are six rows of teeth, two mandibular and four maxillary, with teeth continuously shed and replaced (Mehler and Bennett 2003). The tongue sheath and trachea are identifiable as projections from the floor of the oral cavity.
Petechiation, cyanosis, tacky oral secretions, mucosal inflammation, loss of multiple teeth, haemorrhage, or anatomical asymmetry indicate further evaluation is necessary (Mehler and Bennett 2003). Rostral trauma associated with rubbing on, or colliding with, enclosure walls is uncommon in this species.
Stomatitis or ‘mouth rot’ is a common reason for presentation of captive snakes, and encompasses gingivitis, glossitis, palatitis, and cheilitis from bacterial, fungal, and viral causes. Symptoms may include hyperptyalism, anorexia, dysphagia, loss of teeth, and an irregular appearance to gingiva. Gram-negative bacteria are most commonly isolated from stomatitis cases but there is a significant overlap with commensal bacteria, suggesting external factors may influence development of clinical disease (Rosenthal and Mader 1996). In chronic cases there can be local extension of infection to the nasolacrimal duct, subspectacular space, facial bones, or upper respiratory tract, or distant spread as bacterial emboli or septicaemia. Treatment involves targeted antibiotic therapy based on culture results from lesions, irrigation of lesions using topical antiseptics, and analgesia. Radiographs are useful to determine prognosis and to guide surgical debridement of necrotic or abscessated areas in advanced cases (Mehler and Bennett 2003). For poorly responsive cases histopathology, with particular focus on neoplasia or mycobacteria, and viral screening is advisable.
Ophidian paramyxovirus can result in stomatitis but respiratory and neurological symptoms are more pronounced (Hyndman 2012). Fungal or parasitic stomatitis is rare in captive snakes (Mehler and Bennett 2003).
16.4.7 Ophthalmic Disease
Retained spectacles are the most common ophthalmic abnormality identified in snakes, followed by subspectacular abscess, ocular trauma, and cataracts (Hausmann et al. 2013). Feeding of live prey was found to be a significant cause of ophthalmic trauma and is best avoided.
Subspectacular abscess formation is commonly a consequence of bacterial stomatitis extending along the nasolacrimal duct into the subspectacular space. Colubrid snakes appear more likely to suffer from this condition than other snake families (Hausmann et al. 2013). Animals present with apparent opaque distension of the subspectacular space. The subspectacular space measures approximately 0.14 mm in normal corn snakes (Hollingsworth et al. 2007), but can be increased dramatically with accumulation of purulent material. Systemic antibiotics and anti-inflammatory agents are administered and a 30° wedge resection of the ventral spectacle carried out for drainage and ongoing topical therapy (Cullen et al. 2000). Recurrence is common and may require repeat surgical intervention (Hausmann et al. 2013). Culture of material collected at surgery is advised to guide antibiotic therapy. Staphylococcus spp., Salmonella arizonae and Pseudomonas aeruginosa have been cultured from four cases of subspectacular infections, all presumed to be opportunistic pathogens (Hausmann et al. 2013).
Occasionally an obstruction of the nasolacrimal duct leads to sterile distension of the subspectacular space and treatment involves drainage of the fluid and management of the inflammatory process causing the obstruction. Untreated, these cases can progress to subspectacular abscess formation.
Cataracts have been reported in other colubrids (Martin et al. 1994; Daltry 2006), but appear rare in corn snakes. Phacoemulsification has been used successfully in the closely related Texas rat snake (Ledbetter et al. 2017).
16.4.8 Gastrointestinal Disease
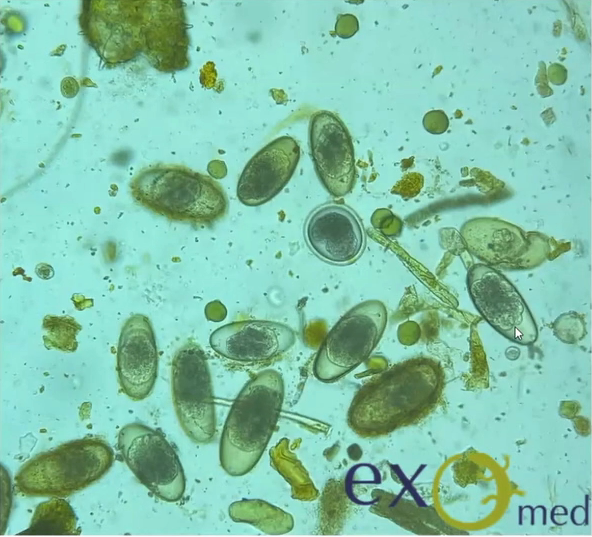
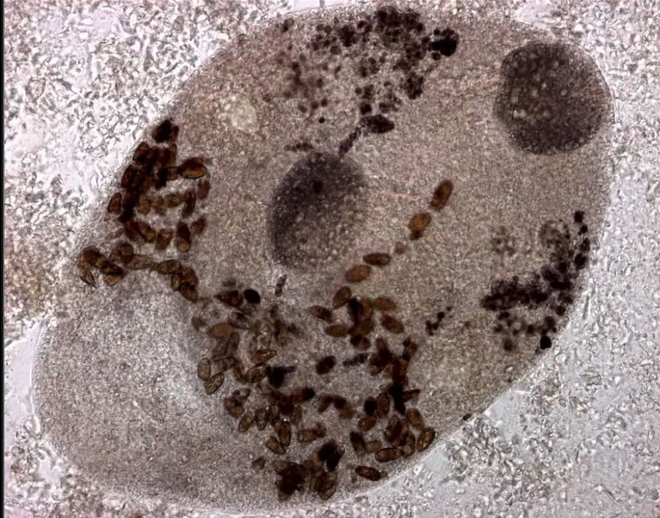
Routine parasite assessment of faecal samples is advisable annually for clinically well snakes, and at presentation for animals presenting for any illness, ideally including testing for Cryptosporidia.
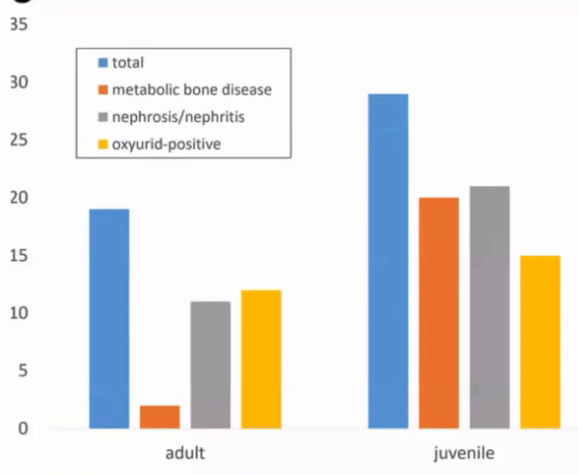
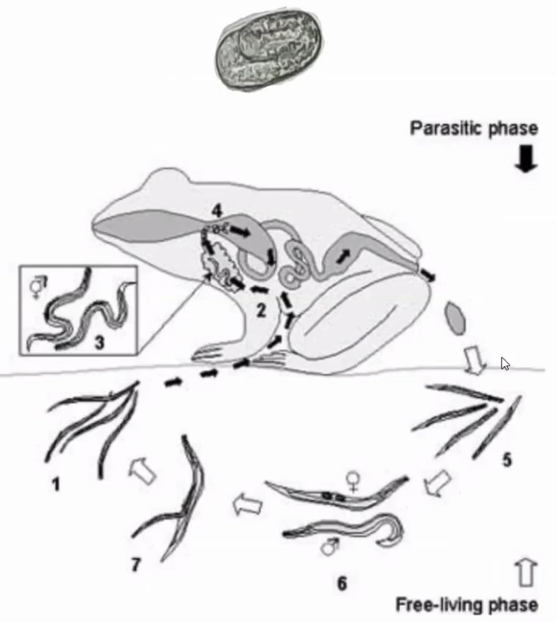
Cryptosporidium coccidia are a significant cause of gastrointestinal disease in reptiles, with Cryptosporidium serpentis recognised as the predominant snake pathogen and Cryptosporidium varanii (syn. Cryptosporidium saurophilum) predominating in lizards, with other species less commonly identified (Xiao et al. 2004; Pavlasek and Ryan 2008; Richter et al. 2011). Oocysts are shed in the faeces of infected animals and remain viable for over a year (Jenkins et al. 1997). Ingestion results in release of sporozoites in the intestinal tract which migrate into the gastric epithelial cells to replicate in snakes (Greiner 2003). Released merozoites can directly infect other epithelial cells and repeat this process, or form gametes to generate oocysts (Greiner 2003). Clinical signs in snakes include regurgitation, gastric distension, weight loss, anorexia, diarrhoea, and incomplete digestion of food (Richter et al. 2011). Chronic gastritis with mucosal hypertrophy, hyperaemia, cobblestone appearance, reduced prominence of rugal folds, and luminal reduction may be noted on endoscopy or post-mortem examination (Cimon et al. 1996; Bercier et al. 2017). Cryptosporidiosis is typically a chronic disease of adult animals, though one case in a oneyear-old corn snake has been reported. In this case, the snake was concurrently infected with adenovirus and it was hypothesised that immunosuppression was a contributing factor to disease development (Mahapatra et al.
2013). Biliary tract cryptosporidiosis and associated cholecystitis has been reported in corn snakes with concurrent intestinal cryptosporidiosis, with no clinical signs of biliary disease (Cimon et al. 1996). A case of oesophageal-gastric-duodenal double compounded intussusception has also been reported as a suspected consequence of cryptosporidiosis in a corn snake (Bercier et al. 2017).
Oocysts are very small and almost transparent so identification on faecal sample flotation can be insensitive. Acidfast staining of samples can increase sensitivity (Greiner 2003). PCR can be performed on faecal samples, gastric wash samples, gastric biopsy, or regurgitated food items and shows greater sensitivity (Yimming et al. 2016). Speciation of cryptosporidia detected by any test is important as Cryptosporidium parvum and Cryptosporidium muris from rodent prey can be present within the intestinal tract following a feed as incidental findings (Greiner 2003). A high detection rate of Cryptosporidia has been reported in corn snakes in one study, with 25% of samples testing positive on PCR (Richter et al. 2011). Interestingly of the 27 positive samples, 17 were C. varanii, one was a similar lizard genotype and the other 9 samples did not achieve a species level diagnosis. In this study it was not determined whether these infections were associated with clinical disease, though other reports suggest that both natural and experimental infection of corn snakes with C. varanii does not appear to result in clinical disease (Xiao et al. 2004; Plutzer and Karanis 2007). Other studies have demonstrated a predominance of C. serpentis, or incidental findings of C. parvum and C. muris, in corn snakes with a positive Cryptosporidium PCR (Xiao et al. 2004; Yimming et al. 2016).
Treatment of infected animals is unlikely to be successful so it is important to quarantine and test new animals prior to introduction into a collection. Weekly testing over a 30 day period has been recommended (Greiner 2003). Halofuginone, spiramycin, and paromomycin have been trialled in snakes but were unsuccessful (Graczyk et al. 1996). Hyperimmune bovine colostrum, has been shown to reduce clinical signs and oocyst shedding in snakes with no side effects, but convincing evidence of clearance of infection is lacking (Graczyk et al. 1998). Complete disinfection of enclosures of infected animals is difficult, as the recommended methods of heat treatment, ammonia-based disinfectants, dessication, and prolonged exposure to ultraviolet light will significantly reduce numbers, but not eliminate viable oocysts (King and Monis 2007).
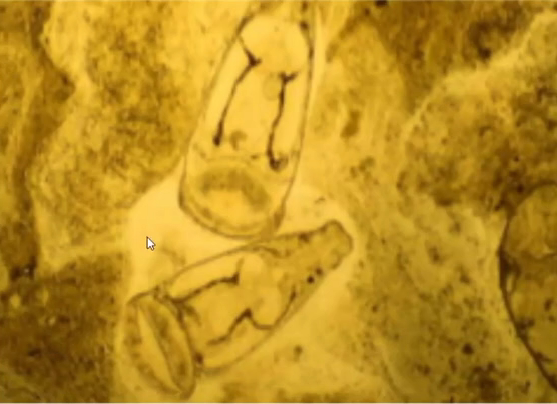
Other endoparasites appear uncommon pathogens in corn snakes. Eimeria oocysts and Ophiostrongylus ova have been identified as co-infections in corn snakes with clinical Cryptosporidiosis (Cimon et al. 1996). Unspecified strongylids were identified in 40% of corn snakes in one study but a wider survey of colubrid snakes found a much lower overall parasitic prevalence with low numbers (<3.3%) testing positive for Kalicephalus, ascarids, heterakids, Rhabdias, Strongyloides, oxyurids, and Balantidium and moderate numbers (7.4%) positive for Nyctotherus (Pasmans et al. 2008; Rataj et al. 2011). Some protozoa may be commensal, but the author has seen clinical signs in colubrid snakes of gas distension of the intestinal tract, foul-smelling liquid faeces, and anorexia in association with high numbers of flagellated motile protozoa on faecal microscopy. Treatment with metronidazole resolves symptoms effectively.
16.4.9 Reproductive Pathology
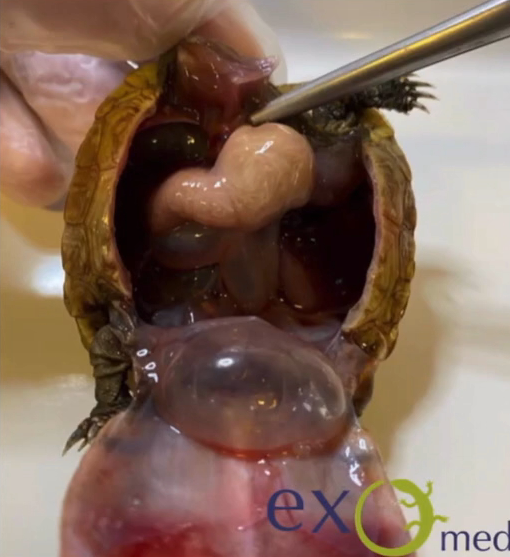
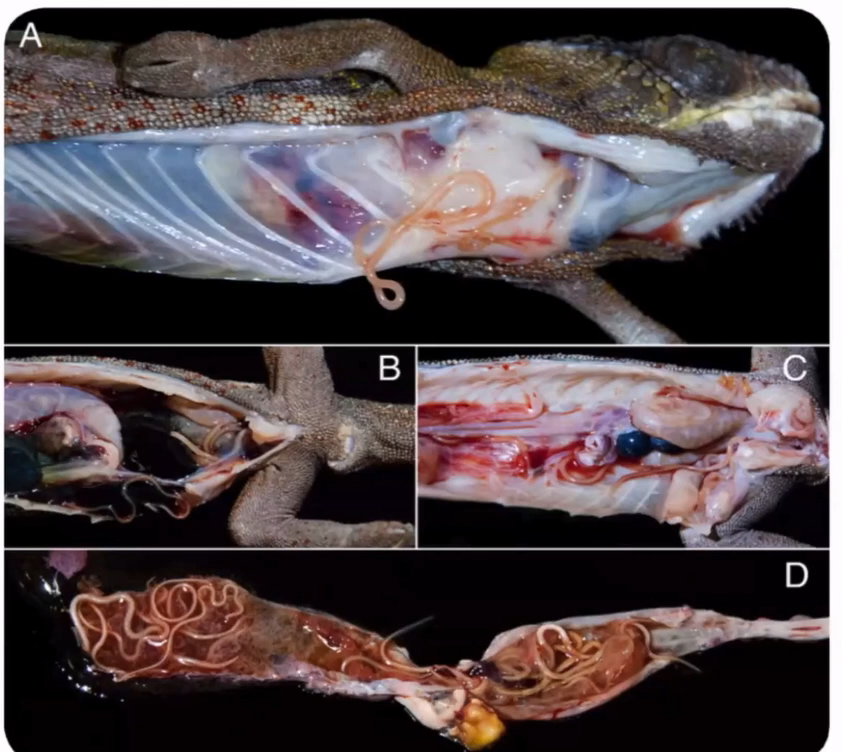
Dystocia is common in snakes, and inciting factors include a lack of a suitable nesting site, social stressors, dehydration, malnutrition, obesity, pathology of the reproductive tract, renomegaly, and oversized or malformed eggs (DeNardo 2006; Sykes 2010). Presenting signs include failure to complete oviposition of a clutch of eggs, prolonged unproductive straining, oviductal prolapse, or non-specific signs such as lethargy and anorexia in an animal beyond the expected date of oviposition (Sykes 2010). Eggs are relatively large and are often visible and palpable along the ventral surface of the caudal coelom, as a series of soft swellings. Gentle palpation may identify abnormally shaped or sized eggs, but care should be taken as the oviduct is thin and highly fragile (Stahl 2002). Imaging can be used to confirm numbers of eggs present as some, notably the smaller infertile eggs (‘slugs’) may not be as readily palpated. If all eggs appear normal in structure then medical therapy using calcium and oxytocin can be considered. This is not appropriate if oviposition started more than 72 hours previously as response will be poor and there is a higher likelihood of adhesion of eggs to the oviduct wall, risking oviductal rupture (Stahl 2002). Arginine vasotocin is more effective than oxytocin but is not currently commercially available (Millichamp et al. 1983).
An alternative to medical therapy is manual removal, or ‘milking’ of eggs, but this typically requires sedation and carries a significant risk of oviductal trauma so is often not an appropriate approach (DeNardo 2006). Eggs are progressively pushed caudally and out of the cloaca by running a thumb firmly in short strokes along the ventral aspect of the snake. If large eggs are not able to be fully exteriorised, the contents may be aspirated through the vent once the egg is visualised, enabling collapse and easier passage. This is often useful where one oversized egg is preventing oviposition of following eggs. Manual removal should not be carried out after oxytocin therapy as this increases the potential for oviductal rupture (Millichamp et al. 1983).
Percutaneous aspiration is an alternative to enable passage of large eggs. Under sterile conditions a wide gauge needle is inserted, between the first and second row of lateral scales, into the egg (Stahl 2002). The egg contents are aspirated and the snake will then normally pass the collapsed egg within 24 hours (Stahl 2002). There is a risk of ongoing leakage of egg contents into the oviduct and the coelom, resulting in salpingitis or coelomitis, particularly with incomplete deflation. Chronically retained eggs tend to have solid contents and cannot be aspirated (Stahl 2002) and adhesed eggs will not progress.
Surgery is preferred if medical therapy, egg manipulation, or ovocentesis has failed, or where adhesion to the oviduct wall is suspected based on chronicity of signs or immobility of one or more eggs (Lock 2000). General anaesthesia is required, and a lateral coeliotomy is carried out. The skin is
Figure 16.9 Use of everting horizontal mattress sutures for skin closure following salpingotomy.
prepared with a dilute iodine solution, and gently scrubbed between scales using a soft brush. The incision is made directly over the egg, two rows of scales dorsal to the gastric scales with the incision following the scale margins (Bercier et al. 2017). The ribs are transected and the coelomic membrane sharply dissected to visualise the egg within the oviduct. An incision is made in the thin oviduct wall and the egg removed. Additional eggs may be manipulated through the same incision, but often multiple skin and oviduct incisions are necessary to remove all eggs. The oviduct is then closed with a continuous inverting pattern using a 4.0–5.0 long-lasting absorbable suture material such as polydioxanone and the process repeated for the contralateral oviduct, through the same skin incisions. If a prolapse, salpingitis, or oviductal rupture is present, salpingectomy and removal of the ipsilateral ovary is advisable but requires multiple incisions in order to ligate associated vessels and remove all tissues (Lock 2000). An everting pattern such as horizontal mattress sutures, or skin staples, is used for skin closure (Figure 16.9) and sutures or staples are removed at six to eight weeks post-surgery.
16.4.10 Neoplasia
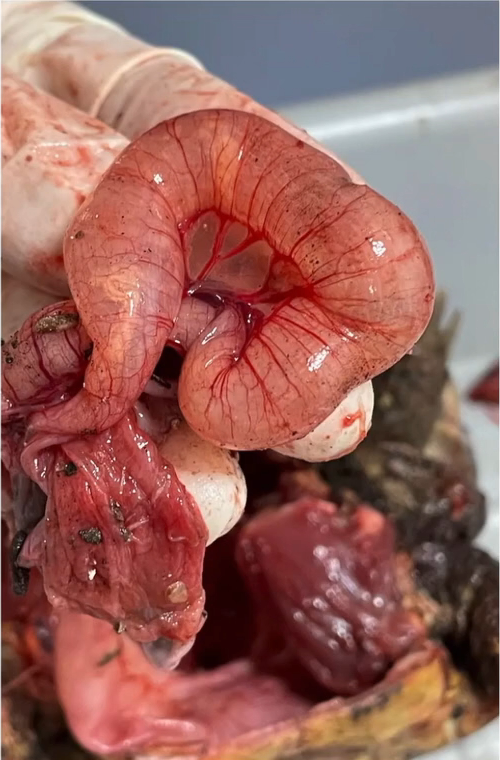
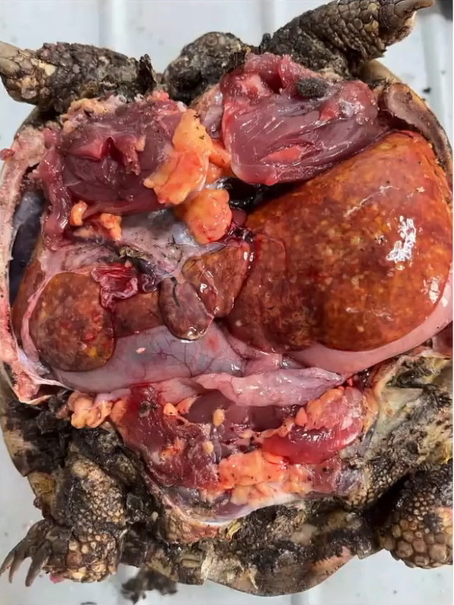
A wide range of case reports of neoplasia have been published for this species, with many affecting young adult snakes of one to five years of age. These are listed in Table 16.3. In the majority of cases a visible swelling is identified (Figure 16.10), though for an oviductal carcinoma, mucopurulent salpingitis without an overt mass was the primary finding (Pereira and Viner 2008).
Table 16.3 Reported neoplasms in corn snakes.
| Neoplasm | Age | Comments | Reference |
|---|---|---|---|
| Subcutaneous lipomas | Various | Reported to be over-represented in corn snakes | Frye (1994), Reavill and Schmidt (2003), Garner et al. (2004), Dietz et al. (2016) |
| Infiltrative lipoma | Adult; 12 yr | Initial fine needle aspiration (FNA) indicative of lipoma in both cases | Burkert et al. (2002), Pinto et al. (2018) |
| Cardiac haemangioma | 3 yr | Associated with atrial wall | Stumpel et al. (2012) |
| Iridophoroma | 22 yr | No metastasis | Muñoz-Gutiérrez et al. (2016) |
| Malignant mixed chromatophoroma | 17 yr | Metastasised | Muñoz-Gutiérrez et al. (2016) |
| Renal adenoma | Adult | Died 3 m post-surgery | Jacobson et al. (1986) |
| Renal cell carcinoma | 8 yr | Amelanistic, anerythristic morph. Metastasised to contralateral kidney, liver, and lung 3 months after nephrectomy | Barten et al. (1994) |
| Renal adenocarcinoma | 5 yr | Metastasised to contralateral kidney 1.5 months after excision | Kao et al. (2016) |
| Oviductal adenocarcinoma | Adult | No distinct mass, oviduct distended by mucopurulent material | Pereira and Viner (2008) |
| Ovarian undifferentiated carcinoma | 6 yr | 9 × 4 cm mass | Petterino et al. (2006) |
| Pulmonary adenocarcinoma | >1.5 yr | No age given, only time on display | Catão-Dias and Nichols (1999) |
| Adrenal adenocarcinoma | >11 yr | No age given, only time on display | Catão-Dias and Nichols (1999) |
| Cloacal adenocarcinoma | >5 yr | No age given, only time on display | Catão-Dias and Nichols (1999) |
| Intestinal adenocarcinoma | Reported as common in corn snakes, one case with metastasis to liver | Garner (2005) | |
| Colonic adenocarcinoma | 3 yr | Multinodular mass at junction of colon and cloaca | Latimer and Rich (1998) |
| Rhabdomyosarcoma | Retrovirus identified in neoplasm | Lunger et al. (1974) | |
| Leiomycosarcoma | >9 yr | Duodenal | Catão-Dias and Nichols (1999) |
| Fibrosarcoma | 12 yr | Suspected invasion into ribs and spine | McNulty and Hoffman (1995) |
| Metastatic chondrosarcoma | 2 yr | Mandibular primary with metastases in the heart, lung, kidney, pancreas, eye | Schmidt and Reavill (2012) |
| Vertebral chondrosarcoma | Three cases arising from vertebral articulations, metastasis noted in one case | Dawe et al. (1980), Garner et al. (1995), Garner (2005) | |
| Splenic haemangiosarcoma | Adult | Died 1.5 m post-surgery | Tuttle et al. (2006) |
| Myeloid leukaemia | >7.5 yr | Multiple organs affected | Catão-Dias and Nichols (1999) |
Figure 16.10 Exploratory coeliotomy for investigation of caudal coelomic mass. An intestinal adenocarcinoma was confirmed on histology (Source: Photo courtesy of Sergio Silvetti).
16.4.11 Neurological Disease
Symptoms of neurological disease in snakes include tremors, seizures, incoordination, blindness, opisthotonus, behavioural changes, obtundation and a failure to feed (Fleming et al. 2003; Mariani 2007). Differential diagnoses include toxin exposure (e.g. nicotine, permethrin), thermal injury, severe renal or hepatic disease, physical trauma, malnutrition, adverse response to medication, viral infection (e.g. Ophidian Paramyxovirus, Ophidian reovirus), bacterial infection of the central nervous system, or hypothermia (Mariani 2007). A case series of three corn snakes with clinical signs and histological findings consistent with the viral Inclusion disease of boids has been reported (Fleming et al. 2003). The report concluded that a similar viral cause may be responsible for the disease in corn snakes but no virus was able to be identified.
16.4.12 Renal Disease
In snakes, the right kidney is located cranial to the left and both have a clear overlapping lobular structure. The primary waste product is uric acid, which is expelled as a white paste with small quantities of clear liquid, and can be post-renally modified limiting the value of urinalysis. Renal compromise may result in anorexia, gout, convulsions, and uncoordinated movements (Divers 2008) but appears uncommon in snakes with only one report of nonneoplastic renal pathology in a corn snake. Giant cell nephritis was described by Zwart (2006), with palpable renal enlargement noted on examination, and histology of the swollen kidneys demonstrating interstitial aggregations of multinucleate giant cells.
16.4.13 Ectoparasitism
Mites are a common finding in captive snakes (Harkewicz 2002). The snake mite (Ophionyssus natricis) causes localised irritation, disruption to skin shedding and may act as a vector for pathogens (Harkewicz 2002). Affected snakes may demonstrate an increased frequency of shedding, increased bathing, and adult mites can be observed on the skin, particularly in skin folds around the eyes and mouth (Harkewicz 2002). Ivermectin or fipronil are preferred for treatment. Permethrins can be used but there are reports of toxicity in snakes (Brooks et al. 1998; Whitehead 2010), and the author has seen multiple cases of neurological symptoms in juvenile corn snakes treated with over the counter permethrin sprays that resolved with supportive treatment.
16.6 ocrdonr
16.5 Preventative Health Measures
No routine vaccinations or parasite treatments are recommended. For new animals, a clinical examination, faecal parasite screen, and husbandry review are advisable. Virology screens should be considered based on risk analysis from the originating collection, and size and type of collection the animal is introduced into. For a single pet animal an exhaustive diagnostic panel is rarely appropriate, but if an animal is being introduced into a large, multispecies or high value collection then there is justification to perform a wider array of pre-movement testing and maintain a longer quarantine period of at least six months. Owners should keep good records detailing feeding and shedding schedules and annual faecal parasitology is a sensible precaution.
16.6 Imaging
Radiography is of particular value for skeletal lesions, uric acid depositions, mineralisation of soft tissues, gastrointestinal foreign bodies, dystocia, effusions, and organomegaly (de la Navarre 2006). However, radiography is insensitive in identification of lower respiratory tract pathology (Schumacher 2003) and the coelomic soft tissues offer poor contrast. Contrast studies using barium have been described to enhance detail, but transit time can be in excess of 72 hours (Banzato et al. 2013).
Ultrasonography has been used to assess reproductive status of female corn snakes, with 8–12 MHz linear transducers used to visualise ovarian follicles. The left ovary is found at a distance of 64–75% along the snout-vent length (SVL), and the right ovary is 70–80% SVL (Divers 2008). Pre-ovulatory follicles have a round, uniform hyperechoic appearance in comparison to previtellogenic, hypoechoic follicles (Oliveri et al. 2018). Ocular ultrasonography is also reported in corn snakes, with ultra-high frequency probes necessary (Hollingsworth et al. 2007).
Echocardiography is valuable for subjective assessment of cardiac anatomy and contractility but no reference ranges for measurements have been documented for this species. The liver (30–50% SVL) and intestinal tract (stomach 48–58% SVL [Divers 2008]) can also be assessed readily using ultrasonography.
Nuclear scintigraphy, using 99mTc-MAG3 provides high quality images of the kidneys in corn snakes and may be a valuable tool in the future given the limitations of other methods of imaging (Sykes IV et al. 2006).
Table: Formulary
| Medication | Dose | Interval | Additional comments |
|---|---|---|---|
| Anaesthesia | |||
| Alfaxalone | 20 mg/kg IM | Inject into cranial half of body, gives 40 mins anaesthesia (James et al. 2018) | |
| Alfaxalone | 5–10 mg/kg IV, IC | Fast onset, 15–20 mins anaesthesia | |
| Propofol | 5–10 mg/kg IV, IC | IV access may not be feasible in all individuals, use lower dose for intracardiac route (Bennett et al. 1998; Stahl | |
| 2002) | |||
| Isoflurane | 2–3% inhaled | Induction may be prolonged. Useful for maintenance at 1–2% (Bertelsen et al. 2005) | |
| Sevoflurane | 4–5% inhaled | Induction may be prolonged. Useful for maintenance at 2–3% (Bertelsen et al. 2005) | |
| Medetomidine and ketamine | 0.15 mg/kg and 10 mg/ kg IM, IV | Useful for sedation, can be partially reversed with atipamezole but recovery may be prolonged (Mosley 2005; Bertelsen 2014) | |
| Metronidazole | 100 mg/kg PO | Two doses, 2 weeks apart | Flagellates (Scullion and Scullion 2009) |
| Fenbendazole | 25 mg/kg PO | Weekly for up to four treatments | Amoebae, flagellates and enteric helminths (Funk and Diethelm 2006) |
| Paromomycin | 300–360 mg/kg PO | q48h for 2 weeks | For Cryptospodiosis, does not eliminate disease in corn snakes (Paré and Barta 1997) |
| Hyperimmune bovine colostrum | 10 ml/kg PO | Weekly for 6 weeks | For Cryptosporidiosis, reduced clinical signs and shedding (Graczyk et al. 1998) |
| Ivermectin | 200 ug/kg SC, IM | repeat after 2 wks | Snake mites (Harkewicz 2002) |
| Analgesia | |||
| Butorphanol | 10 mg/kg SC, IM | q24h | Demonstrated to reduce response to thermal stimulus in one study at 20 mg/kg (Sladky et al. 2008), but respiratory depression possible and this high dose is not advisable (Sladky and Mans 2012) |
| Morphine | 1–5 mg/kg SC, IM | q24h | Effective analgesia in other reptile species. Analgesic efficacy inconsistent in this species (Sladky and Mans |
| 2012) | |||
| Tramadol | 5–10 mg/kg SC, IM, PO | q48h | Extrapolated from other reptile species (Baker et al. 2011) |
| Meloxicam | 0.2–0.3 mg/kg IV, IM, SC, PO | q24–48h | Presumed anti-inflammatory effects (Sladky and Mans 2012) |
| Antibiotics | |||
| Oxytetracycline | 6–10 mg/kg PO, IM, SC | q24h | Injectable preparations can result in localised inflammation (Gibbons et al. 2013) |
| Azithromycin | 10 mg/kg PO | q2–7 days | Based on royal python (Python regius) dose (Coke et al. 2003) |
| Ceftazidime | 20 mg/kg IM, IV | q 48–72h | Used for gram-negative bacterial infections. Third generation cephalosporin, should not be first line antibiotic choice |
| Enrofloxacin | 5–10 mg/kg PO, IM, SC | q24h | Injectable preparations can cause tissue necrosis (Gibbons et al. 2013). Fluoroquinolones should not be first line antibiotics. |
| Terbinafine | 2 mg/ml solution, nebulisation | 30 mins nebulisation daily | To treat fungal dermatosis, including Ophidiomyces ophiodiicola (Kane et al. 2017) |
| Ivermectin | 5 mg/l dilution topically on animals and equipment | repeat after 2 wks | Snake mites (Harkewicz 2002) |
| Fipronil (0.29% spray) | 2 ml/kg topically | q 7–10 days | For ectoparasites, spray or wipe on snake in a wellventilated space, wash off after 5 mins (Fitzgerald and Vera 2006) |
| Miscellaneous | |||
| Furosemide | 2–5 mg/kg IM | q24h | Diuretic for cardiac cases, variable efficacy across species, no data in corn snakes (Selleri and HernandezDivers 2006; Bogan 2017) |
| Pimobendan | 0.2 mg/kg PO | q24h | Extrapolated from lizard dose (Jepson 2009). For dilative cardiomyopathy or congestive heart failure |
| Allopurinol | 20 mg/kg PO | q24h | Decreases uric acid synthesis in renal compromise (Selleri and Hernandez-Divers 2006) |
| Probenecid | 2–4 mg/kg PO | q24h | Increases uric acid excretion, for renal compromise (Selleri and Hernandez-Divers 2006) |
| Methimazole | 2 mg/kg PO | q24h | For hyperthyroidism (Harkewicz 2002) |
| Thyroxine | 0.025 mg/kg PO | q1–5 days | For hypothyroidism (Hunt 2015) |
| Oxytocin | 5–20 iu/kg IM | Start at lower end, repeat with higher dose 6–12 hrs later for maximum of 3 doses | For dystocia (Stahl 2002) |
| Barium 25% | 25 ml/kg PO | For gastrointestinal contrast studies (Banzato et al. 2013) |
Abbas, M.D., Marschang, R.E., Schmidt, V. et al. (2011). A unique novel reptilian paramyxovirus, four atadenovirus types and a reovirus identified in a concurrent infection of a corn snake (Pantherophis guttatus) collection in Germany. Veterinary Microbiology 150 (1–2): 70–79.
Acierno, M.J., Mitchell, M.A., Zachariah, T.T. et al. (2008). Effects of ultraviolet radiation on plasma 25-hydroxyvitamin D3 concentrations in corn snakes (Elaphe guttata). American Journal of Veterinary Research 69 (2): 294–297.
Allender, M.C., Raudabaugh, D.B., Gleason, F.H. et al. (2015). The natural history, ecology, and epidemiology of Ophidiomyces ophiodiicola and its potential impact on free-ranging snake populations. Fungal Ecology 17: 187–196.
Baker, B.B., Sladky, K.K., and Johnson, S.M. (2011). Evaluation of the analgesic effects of oral and subcutaneous tramadol administration in red-eared slider turtles. Journal of the American Veterinary Medical Association 238 (2): 220–227.
Banzato, T., Hellebuyck, T., Van Caelenberg, A. et al. (2013). A review of diagnostic imaging of snakes and lizards. Veterinary Record 173 (2): 43–49.
Barten, S.L., Davis, K., Harris, R.K. et al. (1994). Renal cell carcinoma with metastases in a corn snake (Elaphe guttata). Journal of Zoo and Wildlife Medicine 25 (1): 123–127.
Bartlett, P.P., Griswold, B., and Bartlett, R.D. (2001). Corn snake. In: Reptiles, Amphibians, and Invertebrates: An Identification and Care Guide (eds. P.P. Bartlett, B. Griswold and R.D. Bartlett), 41–42. Hauppage, NY: Barron’s Educational Series.
Beaufrère, H., Schilliger, L., and Pariaut, R. (2016). Cardiovascular system. In: Current Therapy in Exotic Pet Practice (eds. M.A. Mitchell and T.N. Tully), 151–220. St. Louis, MO: Elsevier.
Bellamy, T. and Stephen, I. (2007). The Effect of Ultra-Violet B (UVB) Illumination and Vitamin D3 on the Activity, Behaviour and Growth Rate of the Juvenile Jamaican Boa Epicrates subflavus. Master’s dissertation. University of London, United Kingdom.
Bennett, R.A., Schumacher, J., Hedjazi-Haring, K. et al. (1998). Cardiopulmonary and anesthetic effects of propofol administered intraosseously to green iguanas. Journal of the American Veterinary Medical Association 212 (1): 93–98.
Bercier, M., Zoll, W., Rosenberg, J.F. et al. (2017). Gastric intussusceptions in a red corn snake (Pantherophis guttatus) associated with Cryptosporidiosis. Case Reports in Veterinary Medicine 2017: 4270904.
Bertelsen, M.F. (2014). Squamates (snakes and lizards). In: Zoo Animal and Wildlife Immobilization and Anesthesia, 2e (eds. G. West, D. Heard and N. Caulkett), 351–363. Chichester, UK: Wiley.
Bertelsen, M.F., Mosley, C., Crawshaw, G.J. et al. (2005). Inhalation anesthesia in Dumeril’s monitor (Varanus dumerili) with isoflurane, sevoflurane, and nitrous oxide: effects of inspired gases on induction and recovery. Journal of Zoo and Wildlife Medicine 36 (1): 62–68.
Blackburn, D.G. and Flemming, A.F. (2009). Morphology, development, and evolution of fetal membranes and placentation in squamate reptiles. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 312 (6): 579–589.
Bogan, J.E. Jr. (2017). Ophidian cardiology – a review. Journal of Herpetological Medicine and Surgery 27 (1–2): 62–77.
Bontrager, L.R., Jones, D.M., and Sievert, L.M. (2006). Influence of meal size on postprandial thermophily in cornsnakes (Elaphe guttata). Transactions of the Kansas Academy of Science 109 (3): 184–190.
Brames, H. (2007). Aspects of light and reptilian immunity. Iguana 14 (1): 19–23.
Brooks, J.E., Savarie, P.J., and Johnston, J.J. (1998). The oral and dermal toxicity of selected chemicals to brown tree snakes (Boiga irregularis). Wildlife Research 25 (4): 427–435.
Bull, J.J. (1980). Sex determination in reptiles. The Quarterly Review of Biology 55 (1): 3–21.
Burbrink, F.T. (2002). Phylogeographic analysis of the cornsnake (Elaphe guttata) complex as inferred from maximum likelihood and Bayesian analyses. Molecular Phylogenetics and Evolution 25 (3): 465–476.
Burkert, B.A., Tully, T.N., Nevarez, J. et al. (2002). Infiltrative lipoma in a corn snake, Elaphe guttata guttata. Journal of Herpetological Medicine and Surgery 12 (3): 33–35.
Bush, M. and Smeller, J. (1978). Blood collection and injection techniques in snakes. Veterinary Medicine, Small Animal Clinician 73 (2): 211–214.
Calvert, I. (2004). Nutritional problems. In: BSAVA Manual of Reptiles (eds. S.J. Girling and P. Raiti), 289–308. Gloucester, UK: British Small Animal Veterinary Association.
Catão-Dias, J.L. and Nichols, D.K. (1999). Neoplasia in snakes at the National Zoological Park, Washington, DC (1978–1997). Journal of Comparative Pathology 1 (120): 89–95.
Chiu, K.W. and Lynn, W.G. (1970). The role of the thyroid in skin-shedding in the shovel-nosed snake, Chionactis occipitalis. General and Comparative Endocrinology 14: 467–474.
Chiu, K.W., Leung, M.S., and Maderson, P.F.A. (1983). Thyroid and skin-shedding in the rat snake (Ptyas korros). The Journal of Experimental Zoology 225 (3): 407–410.
Cimon, K.Y., Oberst, R.D., Upton, S.J. et al. (1996). Biliary cryptosporidiosis in two corn snakes (Elaphe guttata). Journal of Veterinary Diagnostic Investigation 8 (3): 398–399.
Coke, R.L., Hunter, R.P., Isaza, R. et al. (2003). Pharmacokinetics and tissue concentrations of azithromycin in ball pythons (Python regius). American Journal of Veterinary Research 64 (2): 225–228.
Cray, C. and Zaias, J. (2004). Laboratory procedures. The Veterinary Clinics of North America. Exotic Animal Practice 7 (2): 487–518.
Crocker-Buta, S.P. and Secor, S.M. (2014). Determinants and repeatability of the specific dynamic response of the corn snake, Pantherophis guttatus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 169: 60–69.
Cullen, C.L., Wheler, C., and Grahn, B.H. (2000). Diagnostic ophthalmology. Bullous spectaculopathy in a king snake. The Canadian Veterinary Journal 41 (4): 327.
Daltry, J.C. (2006). The effect of black rat Rattus rattus control on the population of the Antiguan racer snake Alsophis antiguae on Great Bird Island, Antigua. Conservation Evidence 3: 30–32.
Dawe, C.J., Small, J.D., Banfield, W.G. et al. (1980). Chondrosarcoma of a corn snake (Elaphe guttata) and nephrobastoma of a rainbow trout (Salmo gairdneri) in cell culture. In: The Comparative Pathology of Zoo Animals (eds. R. Montali and G. Migaki), 603–612. Washington, DC: Smithsonian Institute Press.
DeNardo, D. (2006). Dystocias. In: Reptile Medicine and Surgery, 2e (ed. D. Mader), 787–792. St. Louis, MO: Elsevier Inc.
Dietz, J., Heckers, K.O., Aupperle, H. et al. (2016). Cutaneous and subcutaneous soft tissue tumours in snakes: a retrospective study of 33 cases. Journal of Comparative Pathology 155 (1): 76–87.
Divers, S. (2008). Snake radiology: the essentials. Proceedings of the North American Veterinary Conference, 19–23 January, Orlando, FL: 1772–1774.
van Doorn, K. and Sivak, J.G. (2013). Blood flow dynamics in the snake spectacle. Journal of Experimental Biology 216 (22): 4190–4195.
Echternacht, A. and Hammerson, G.A. (2016). Pantherophis guttatus. The IUCN Red List of Threatened Species 2016: e.T63863A71740603. http://dx.doi.org/10.2305/IUCN. UK.2016-3.RLTS.T63863A71740603.en. (accessed 29 November 2018).
Fahrig, B.M., Mitchell, M.A., Eilts, B.E. et al. (2007). Characterization and cooled storage of semen from corn snakes (Elaphe guttata). Journal of Zoo and Wildlife Medicine 38 (1): 7–12.
Farrell, A.P., Gamperl, A.K., and Francis, E.T.B. (1998). Comparative aspects of heart morphology. In: Biology of the Reptilia, vol. 19 (Morphology G) (eds. C. Gans and A.S. Gaunt), 375–424. Ithaca, NY: Society for the Study of Amphibians and Reptiles.
Fisher, P.L. and Csurhes, S. (2009). Pest Animal Risk Assessment: American Corn Snake Elaphe guttata. Brisbane: Queensland Primary Industries and Fisheries.
Fitzgerald, K.T. and Vera, R. (2006). Acariasis. In: Reptile Medicine and Surgery, 2e (ed. D.R. Mader), 728. St. Louis, MO: Saunders, Elsevier.
Fleming, G.J., Heard, D.J., Jacobson, E.R. et al. (2003). Cytoplasmic inclusions in corn snakes, Elaphe guttata, resembling inclusion body disease of boid snakes. Journal of Herpetological Medicine and Surgery 13 (2): 18–22.
Fonseca, É., Marques, R., and Tinôco, M.S. (2014). New records of Pantherophis guttatus (Squamata: Colubridae) in the state of Bahia, an alien species to Brazil. Salamandra 50: 241–244.
Frye, F.L. (1994). Reptile Clinician’s Handbook: A Compact Clinical and Surgical Reference. Malabar, FL: Krieger Publishing Company.
Funk, R.S. (1996). Biology: snakes. In: Reptile Medicine and Surgery (ed. D.R. Mader), 39–46. Philidelphia, PA: W. B. Saunders Co.
Funk, R.S. and Diethelm, G. (2006). Reptile formulary. In: Reptile Medicine and Surgery, 2e (ed. D.R. Mader), 1119–1139. St. Louis, MO: Saunders Elsevier.
Garner, M.M. (2005). Trends in reptilian neoplasia: a diagnostian’s perspective. Proceedings of the North American Veterinary Conference, 8–12 January, Orlando, FL: 1278–1280
Garner, M.M., Collins, D., and Joslin, J. (1995). Vertebral chondrosarcoma in a corn snake. Proceedings of Annual Conference AAZV, August: 332–333.
Garner, M.M., Hernandez-Divers, S.M., and Raymond, J.T. (2004). Reptile neoplasia: a retrospective study of case submissions to a specialty diagnostic service. Veterinary Clinics: Exotic Animal Practice 7 (3): 653–671.
Gaspar, C. and Dulman, O.M. (2014). Observations regarding the accommodation and feeding of leisure reptiles. Lucrari Stiintifice, seria Medicină Veterinară 57 (3–4): 260.
Gibbons, P.M. (2009). Critical care nutrition and fluid therapy in reptiles. Proceedings of the 15th Annual International Veterinary Emergency & Critical Care Symposium: 91–94.
Gibbons, J.W. and Dorcas, M.E. (2005). Snakes of the Southeast. Athens, GA: University of Georgia Press. Gibbons, P., Klaphake, E., and Carpenter, J.W. (2013). Reptiles. In: Exotic Animal Formulary, 4e (eds. J.W. Carpenter and C.J. Marion), 83–182. St. Louis, MO: Saunders.
Graczyk, T.K., Cranfield, M.R., and Hill, S.L. (1996). Therapeutic efficacy of halofuginone and spiramycin treatment against Cryptosporidium serpentis (Apicomplexa: Cryptosporidiidae) infections in captive snakes. Parasitology Research 82 (2): 143–148.
Graczyk, T.K., Cranfield, M.R., Helmer, P. et al. (1998). Therapeutic efficacy of hyperimmune bovine colostrum treatment against clinical and subclinical Cryptosporidium serpentis infections in captive snakes. Veterinary Parasitology 74 (2–4): 123–132.
Greenacre, C.B., Young, D.W., Behrend, E.N. et al. (2001). Validation of a novel high sensitivity radioimmunoassay procedure for measurement of total thyroxine concentration in psittacine birds and snakes. American Journal of Veterinary Research 62 (11): 1750–1754.
Gregory, P.T., Crampton, L.H., and Skebo, K.M. (1999). Conflicts and interactions among reproduction, thermoregulation and feeding in viviparous reptiles: are gravid snakes anorexic? Journal of Zoology 248 (2): 231–241.
Greiner, E.C. (2003). Coccidiosis in reptiles. Seminars in Avian and Exotic Pet Medicine 12 (1): 49–56.
Harkewicz, K.A. (2002). Dermatologic problems of reptiles. Seminars in Avian and Exotic Pet Medicine 11 (3): 151–161. Hausmann, J.C., Hollingsworth, S.R., Hawkins, M.G. et al. (2013). Distribution and outcome of ocular lesions in snakes examined at a veterinary teaching hospital: 67 cases (1985–2010). Journal of the American Veterinary Medical Association 243 (2): 252–260.
Hollingsworth, S.R., Holmberg, B.J., Strunk, A. et al. (2007). Comparison of ophthalmic measurements obtained via high-frequency ultrasound imaging in four species of snakes. American Journal of Veterinary Research 68 (10): 1111–1114.
Hoppmann, E. and Barron, H.W. (2007). Dermatology in reptiles. Journal of Exotic Pet Medicine 16 (4): 210–224.
Hunt, C. (2015). Thyroid adenocarcinoma in a Garter snake (Thamnophis marcianus). Proceedings of BVZS Spring meeting, Loughborough, UK: 38.
Hyndman, T. (2012). Paramyxoviruses in Australian snakes. Doctoral dissertation, Murdoch University, https:// researchrepository.murdoch.edu.au/id/eprint/10648 (accessed 15 April 2019).
Jacobson, E.R. and Origgi, F. (2002). Use of serology in reptile medicine. Seminars in Avian and Exotic Pet Medicine 11 (1): 33–45.
Jacobson, E.R., Long, P.H., Miller, R.E. et al. (1986). Renal neoplasia of snakes. Journal of the American Veterinary Medical Association 189 (9): 1134–1136.
Jakobsen, S.L., Williams, C.J., Wang, T. et al. (2017). The influence of mechanical ventilation on physiological parameters in ball pythons (Python regius). Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology 207: 30–35.
James, L.E., Williams, C.J., Bertelsen, M.F. et al. (2018). Anaesthetic induction with alfaxalone in the ball python (Python regius): dose response and effect of injection site. Veterinary Anaesthesia and Analgesia 45 (3): 329–337.
Jenkins, M.B., Anguish, L.J., Bowman, D.D. et al. (1997). Assessment of a dye permeability assay for determination of inactivation rates of Cryptosporidium parvum oocysts. Applied and Environmental Microbiology 63 (10): 3844–3850.
Jensen, B., Boukens, B., Wang, T. et al. (2014). Evolution of the sinus venosus from fish to human. Journal of Cardiovascular Development and Disease 1 (1): 14–28. Jepson, L. (2009). Exotic Animal Medicine: A Quick Reference Guide. London, UK: WB Saunders.
Jose, S. (2006). Resident snakes common in other habitats. In: The Longleaf Pine Ecosystem: Ecology, Silviculture, and Restoration (eds. S. Jose, E.J. Jokela and D.L. Miller), 172–173. New York: Springer.
Kane, L.P., Allender, M.C., Archer, G. et al. (2017). Pharmacokinetics of nebulized and subcutaneously implanted terbinafine in cottonmouths (Agkistrodon piscivorus). Journal of Veterinary Pharmacology and Therapeutics 40 (5): 575–579.
Kao, C.F., Chen, J.L., Tsao, W.T. et al. (2016). A renal adenocarcinoma in a corn snake (Pantherophis guttatus) resembling human collecting duct carcinoma. Journal of Veterinary Diagnostic Investigation 28 (5): 599–603.
Kik, M.J. and Mitchell, M.A. (2005). Reptile cardiology: a review of anatomy and physiology, diagnostic approaches, and clinical disease. Seminars in Avian and Exotic Pet Medicine 14 (1): 52–60.
King, B.J. and Monis, P.T. (2007). Critical processes affecting Cryptosporidium oocyst survival in the environment. Parasitology 134 (3): 309–323.
Latimer, K.S. and Rich, G.A. (1998). Colonic adenocarcinoma in a corn snake (Elaphe guttata guttata). Journal of Zoo and Wildlife Medicine: Official Publication of the American Association of Zoo Veterinarians 29 (3): 344–346.
Leary, S., Underwood, W., Anthony, R. et al. (2013). AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. Schaumburg, IL: American Veterinary Medical Association.
Ledbetter, E.C., de Matos, R., Riedel, R.M. et al. (2017). Phacoemulsification of bilateral mature cataracts in a Texas rat snake (Elaphe obsoleta lindheimeri). Journal of the American Veterinary Medical Association 251 (11): 1318–1323.
Lindemann, D.M., Allender, M.C., Rzadkowska, M. et al. (2017). Pharmacokinetics, efficacy, and safety of voriconazole and itraconazole in healthy cottonmouths (Agkistrodon piscivorus) and massasauga rattlesnakes (Sistrurus catenatus) with snake fungal disease. Journal of Zoo and Wildlife Medicine 48 (3): 757–766.
Lock, B.A. (2000). Reproductive surgery in reptiles. The Veterinary Clinics of North America. Exotic Animal Practice 3 (3): 733–752.
Lorch, J.M., Lankton, J., Werner, K. et al. (2015). Experimental infection of snakes with Ophidiomyces ophiodiicola causes pathological changes that typify snake fungal disease. MBio 6 (6) https://doi.org/10.1128/ mBio.01534-15.
Lunger, P.D., Hardy, W.D. Jr., and Clark, F. (1974). C-type virus particles in a reptilian tumor. Journal of the National Cancer Institute 52 (4): 1231–1235.
Maclean, B. and Raiti, P. (2004). Emergency care. In: BSAVA Manual of Reptiles (eds. S.J. Girling and P. Raiti), 65–66. Gloucester, UK: British Small Animal Veterinary Association.
Mader, D. (2002). Treating burns in reptiles, Proceedings TUFTS Animal Expo, Boston, MA.
Mahapatra, D., Reinhard, M., and Naikare, H.K. (2013). Adenovirus and cryptosporidium co-infection in a corn snake (Elaphae guttata guttata). Journal of Zoo and Wildlife Medicine 44 (1): 220–224.
Mans, C. and Braun, J. (2014). Update on common nutritional disorders of captive reptiles. Veterinary Clinics: Exotic Animal Practice 17 (3): 369–395.
Mariani, C.L. (2007). The neurologic examination and neurodiagnostic techniques for reptiles. The Veterinary Clinics of North America. Exotic Animal Practice 10 (3): 855–891.
Martin, J.C., Schelling, S.H., and Pokras, M.A. (1994). Gastric adenocarcinoma in a Florida indigo snake (Drymarchon corais couperi). Journal of Zoo and Wildlife Medicine 25 (1): 133–137.
Martinez-Silvestre, A., Mateo, J.A., and Pether, J. (2003). Electrocardiographic parameters in the Gomeran giant lizard, Gallotia bravoana. Journal of Herpetological Medicine and Surgery 13 (3): 22–26.
Mattison, C. (2006). Snake – The Essential Visual Guide to the World of Snakes. New York: DK Publishing.
McNulty, E. and Hoffman, R. (1995). Fibrosarcoma in a corn snake, Elaphe guttata. Bulletin of the Association of Reptilian and Amphibian Veterinarians 5 (3): 7–8.
Mehler, S.J. and Bennett, R.A. (2003). Oral, dental, and beak disorders of reptiles. The Veterinary Clinics of North America. Exotic Animal Practice 6 (3): 477–503.
Millichamp, N.J., Lawrence, K., Jacobson, E.R. et al. (1983). Egg retention in snakes. Journal of the American Veterinary Medical Association 183 (11): 1213.
Mitchell, M.A. (2004). Snake care and husbandry. The Veterinary Clinics of North America. Exotic Animal Practice 7 (2): 421–446.
Mitchell, M.A. (2009). Reptile cardiology. The Veterinary Clinics of North America. Exotic Animal Practice 12 (1): 65–79.
Mosley, C.A. (2005). Anesthesia and analgesia in reptiles. Seminars in Avian and Exotic Pet Medicine 14 (4): 243–262. Muñoz-Gutiérrez, J.F., Garner, M.M., and Kiupel, M. (2016). Cutaneous chromatophoromas in captive snakes. Veterinary Pathology 53 (6): 1213–1219.
Murray, M.J. (2005). Pneumonia and lower respiratory tract diseases. In: Reptile Medicine and Surgery, 2e (ed. D.R. Mader), 865–877. St. Louis, MO: Saunders.
Nail, A. (2008). Does exposure to UVB light influence the growth rates and behaviour of hatchling Corn Snakes, Pantherophis guttatus? BI6154–Dissertation at Reaseheath College. de la Navarre, B.J. (2006). Common procedures in reptiles and amphibians. Veterinary Clinics: Exotic Animal Practice 9 (2): 237–267.
Oliveri, M., Bartoskova, A., Spadola, F. et al. (2018). Method of semen collection and artificial insemination in snakes. Journal of Exotic Pet Medicine 27 (2): 75–80.
Paré, J.A. and Barta, J.R. (1997). Treatment of cryptosporidiosis in Gila monsters (Heloderma suspectum) with paromomycin. Proceedings of the Association of Reptilian and Amphibian Veterinarians: 23.
Pasmans, F., Blahak, S., Martel, A. et al. (2008). Introducing reptiles into a captive collection: the role of the veterinarian. The Veterinary Journal 175 (1): 53–68.
Pavlasek, I. and Ryan, U. (2008). Cryptosporidium varanii takes precedence over C. saurophilum. Experimental Parasitology 118 (3): 434–437.
Pees, M., Neul, A., Müller, K. et al. (2016). Virus distribution and detection in corn snakes (Pantherophis guttatus) after experimental infection with three different ferlavirus strains. Veterinary Microbiology 182: 213–222.
Penning, D.A. (2012). Growth rates and prey-handling behavior of hatchling corn snakes Pantherophis guttatus (Colubridae). Doctoral dissertation, University of Central Missouri.
Pereira, M.E. and Viner, T.C. (2008). Oviduct adenocarcinoma in some species of captive snakes. Veterinary Pathology 45 (5): 693–697.
Petterino, C., Bedin, M., Podestà, G. et al. (2006). Undifferentiated tumor in the ovary of a corn snake (Elaphe guttata guttata). Veterinary Clinical Pathology 35 (1): 95–100.
Pinto, F.F., Craveiro, H., Marrinhas, C. et al. (2018). What is your diagnosis? Multiple masses in a corn Snake (Pantherophis guttatus). Veterinary Clinical Pathology: 1–3. https://doi.org/10.1111/vcp.12687.
Plutzer, J. and Karanis, P. (2007). Molecular identification of a Cryptosporidium saurophilum from corn snake (Elaphe guttata guttata). Parasitology Research 101 (4): 1141–1145.
Rajeev, S., Sutton, D.A., Wickes, B.L. et al. (2009). Isolation and characterization of a new fungal species, Chrysosporium ophiodiicola, from a mycotic granuloma of a black rat snake (Elaphe obsoleta obsoleta). Journal of Clinical Microbiology 47 (4): 1264–1268.
Ramsay, E.C. and Dotson, T.K. (1995). Tissue and serum enzyme activities in the yellow rat snake (Elaphe obsoleta quadrivitatta). American Journal of Veterinary Research 56 (4): 423–428.
Rataj, A.V., Lindtner-Knific, R., Vlahović, K. et al. (2011). Parasites in pet reptiles. Acta Veterinaria Scandinavica 53 (1): 33.
Reavill, D. and Schmidt, R. (2003). Lipomas in corn snakes (Elaphe guttata guttata): a series of four cases. Proceedings of the Annual Conference of the Association Reptilian and Amphibian Veterinarians.
Richter, B., Nedorost, N., Maderner, A. et al. (2011). Detection of Cryptosporidium species in feces or gastric contents from snakes and lizards as determined by polymerase chain reaction analysis and partial sequencing of the 18S ribosomal RNA gene. Journal of Veterinary Diagnostic Investigation 23 (3): 430–435.
Roark, A.W. and Dorcas, M.E. (2000). Regional body temperature variation in corn snakes measured using temperature-sensitive passive integrated transponders. Journal of Herpetology 34 (3): 481–485.
Rosenthal, K.L. and Mader, D.R. (1996). Special topics: microbiology. In: Reptile Medicine and Surgery (ed. D.R. Mader), 119–125. Philadelphia: WB Saunders.
Rzadkowska, M., Allender, M.C., O’Dell, M. et al. (2016). Evaluation of common disinfectants effective against Ophidiomyces ophiodiicola, the causative agent of snake fungal disease. Journal of Wildlife Diseases 52 (3): 759–762.
Schilliger, L., Tessier, D., Pouchelon, J.L. et al. (2006). Proposed standardization of the two-dimensional echocardiographic examination in snakes. Journal of Herpetological Medicine and Surgery 16 (3): 76–87.
Schmidt, R.E. and Reavill, D.R. (2012). Metastatic chondrosarcoma in a corn snake (Pantherophis guttatus). Journal of Herpetological Medicine and Surgery 22 (3): 67–69.
Schumacher, J. (2003). Reptile respiratory medicine. The Veterinary Clinics of North America. Exotic Animal Practice 6 (1): 213–231.
Scullion, F.T. and Scullion, M.G. (2009). Gastrointestinal protozoal diseases in reptiles. Journal of Exotic Pet Medicine 18 (4): 266–278.
Selleri, P. and Hernandez-Divers, S.J. (2006). Renal diseases of reptiles. Veterinary Clinics: Exotic Animal Practice 9 (1): 161–174.
Sievert, L.M., Jones, D.M., and Puckett, M.W. (2005). Postprandial thermophily, transit rate, and digestive efficiency of juvenile cornsnakes, Pantherophis guttatus. Journal of Thermal Biology 30 (5): 354–359.
Sigler, L., Hambleton, S., and Paré, J.A. (2013). Molecular characterization of reptile pathogens currently known as members of the Chrysosporium anamorph of Nannizziopsis vriesii complex and relationship with some humanassociated isolates. Journal of Clinical Microbiology 51 (10): 3338–3357.
Sladky, K.K. and Mans, C. (2012). Clinical anesthesia in reptiles. Journal of Exotic Pet Medicine 21 (1): 17–31. Sladky, K.K., Kinney, M.E., and Johnson, S.M. (2008). Analgesic efficacy of butorphanol and morphine in bearded dragons and corn snakes. Journal of the American Veterinary Medical Association 233 (2): 267–273.
Smith, D.A., Barker, I.K., and Allen, O.B. (1988). The effect of ambient temperature and type of wound on healing of cutaneous wounds in the common garter snake (Thamnophis sirtalis). Canadian Journal of Veterinary Research 52 (1): 120.
Stahl, S.J. (2002). Veterinary management of snake reproduction. Veterinary Clinics: Exotic Animal Practice 5 (3): 615–636.
Stahlschmidt, Z.R., French, S.S., Ahn, A. et al. (2017). A simulated heat wave has diverse effects on immune function and oxidative physiology in the corn snake (Pantherophis guttatus). Physiological and Biochemical Zoology 90 (4): 434–444.
Stumpel, J.B.G., Del-Pozo, J., French, A. et al. (2012). Cardiac hemangioma in a corn snake (Pantherophis guttatus). Journal of Zoo and Wildlife Medicine 43 (2): 360–366.
Sykes, J.M. (2010). Updates and practical approaches to reproductive disorders in reptiles. Veterinary Clinics: Exotic Animal Practice 13 (3): 349–373.
Sykes, J.M. IV, Schumacher, J., Avenell, J. et al. (2006). Preliminary evaluation of 99mTechnetium diethylenetriamine pentaacetic acid, 99mTechnetium dimercaptosuccinic acid, and 99mTechnetium mercaptoacetyltriglycine for renal scintigraphy in corn snakes (Elaphe guttata guttata). Veterinary Radiology & Ultrasound 47 (2): 222–227.
Tuttle, A.D., Harms, C.A., Van Wettere, A.J. et al. (2006). Splenic hemangiosarcoma in a corn snake, Elaphe guttata. Journal of Herpetological Medicine and Surgery 16 (4): 140–143.
Utiger, U., Helfenberger, N., Schätti, B. et al. (2002). Molecular systematics and phylogeny of Old and New World ratsnakes, Elaphe Auct., and related genera (Reptilia, Squamata, Colubridae). Russian Journal of Herpetology 9 (2): 105–124.
Werler, J.E. and Dixon, J.R. (2004). Corn snake. In: Texas Snakes: Identification, Distribution and Natural History (eds. J.E. Werler, J.R. Dixon and R. Levoy), 107–111. Austin, TX: University of Texas Press.
Whitehead, M. (2010). Permethrin toxicity in exotic pets. The Veterinary Record 166 (10): 306.
Whiteside, D.P. and Gamer, M.M. (2001). Thyroid adenocarcinoma in a crocodile lizard, Shinisaurus crocodilurus. Journal of Herpetological Medicine and Surgery 11 (1): 13–16.
Worthington-Hill, J.O., Yarnell, R.W., and Gentle, L.K. (2014). Eliciting a predatory response in the eastern corn snake (Pantherophis guttatus) using live and inanimate sensory stimuli: implications for managing invasive populations. International Journal of Pest Management 60 (3): 180–186.
Xiao, L., Ryan, U.M., Graczyk, T.K. et al. (2004). Genetic diversity of Cryptosporidium spp. in captive reptiles. Applied and Environmental Microbiology 70 (2): 891–899.
Yimming, B., Pattanatanang, K., Sanyathitiseree, P. et al. (2016). Molecular identification of Cryptosporidium species from pet snakes in Thailand. The Korean Journal of Parasitology 54 (4): 423.
Zwart, P. (2006). Renal pathology in reptiles. The Veterinary Clinics of North America. Exotic Animal Practice 9 (1): 129–159.
- 尽快去医院。如果你无法确定该不该去医院,那就去。除非确实没有条件时再使用替代方法。(此处没有条件指的是像新冠疫情管控之类的不可抗力,不包括:没钱、太远、不熟悉宠物医院在哪儿等等不是理由的理由。)
- 如果你已经知道是什么疾病,可以提前查阅(正儿八经的)兽医教科书和文献进一步判断,有助于与兽医交流并判断出不可靠的兽医。
- 可找靠谱的渠道在线问诊
- 花钱的那种,别去给人发私信像免费使用别人的知识和经验;
- 有的宠物医院的网站或公众号有在线问诊功能;有些兽医会开微博问答;有的医院先电话联系时可以主动询问能否把图/视频发过去做初步判断;
- 在“信息来源”一章列出了个人接触过的部分医生(但我仅能利用读到的更高等级证据时判断哪些医生不好,但没有能力完全判断哪些医生好,只能说“尚未发现不好”)
- 不要“上网查”(即不要用非学术的网页搜索引擎、搜各种无法证实可信度的网站上的信息,各种场合下的“网友回复”只能作为进一步检索的线索,不能直接作为依据)。
DVM: Doctor of Veterinary Medicine
BVS
BVSc
BVMS
BVM
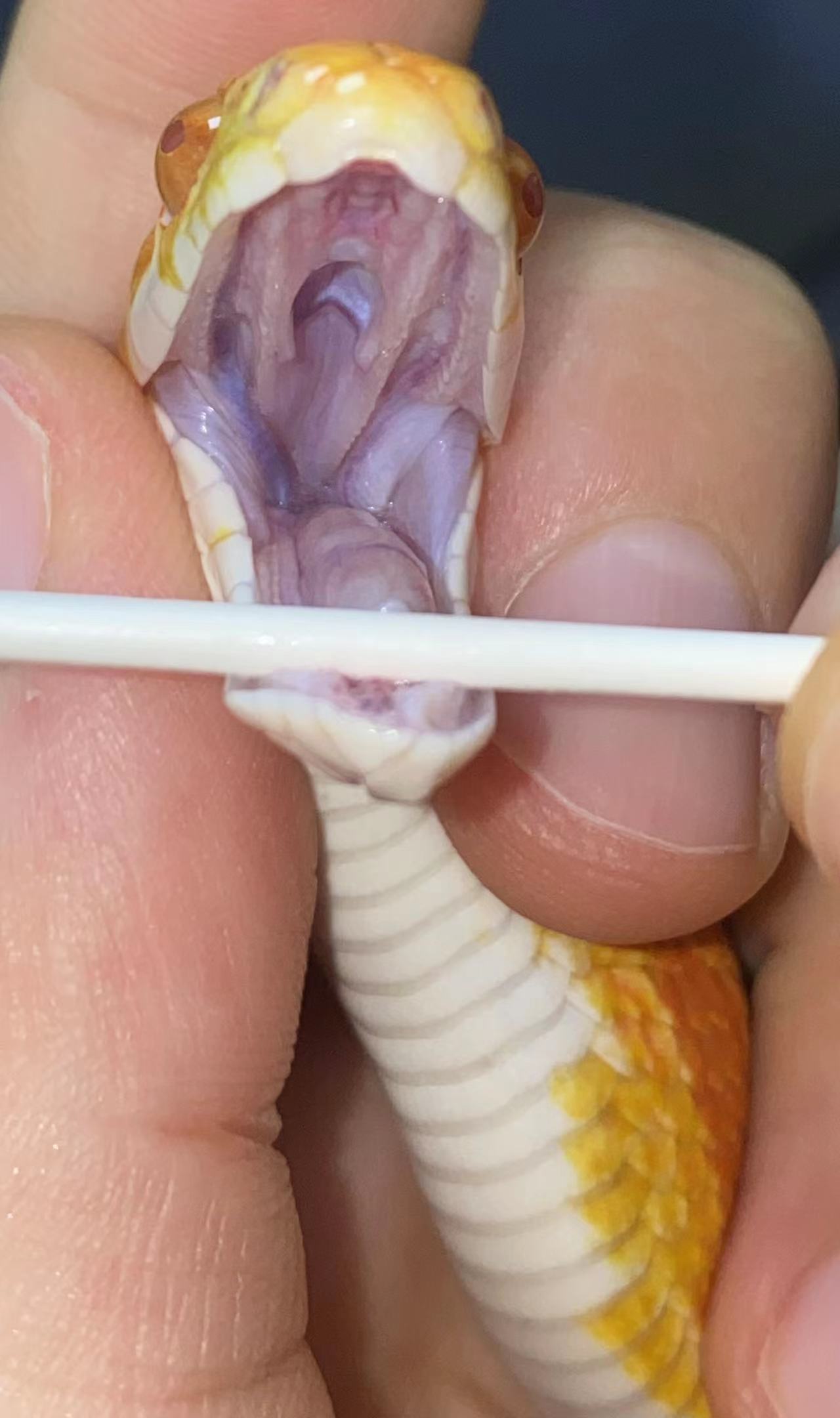
许多头部疾病、口腔疾病(如眼镜卡皮、鼻孔卡皮、口腔炎、呼吸道感染等)需控制蛇头仔细观察,这也是查体的重要环节。许多玉米蛇抗拒被触碰头部,导致没法观察、没法拍照。因此需要学习控制蛇头的正确方法。
一般来说,手的拇指和中指用来支撑头部的侧面,手指位置在枕骨(occiput)后方。食指放在头顶上。用另一只手用来支撑蛇的身体。通过这种方式固定蛇的头部,可以给craniumcervical
junction提供更大的支撑,蛇只有一个occiput,操作不当容易脱臼。
(Mader, 2019 ![]() )IV
)IV
由专业兽医演示的控制蛇的头部进行口腔检查的手法:
应当注意提供对脊椎得支撑,放置蛇头被控制时挣扎导致脊椎损伤。
https://www.bilibili.com/video/BV1CJ4m1b7Bz/?spm_id_from=333.999.0.0
- 信息来源:
- S. Divers, S. Stahl, Mader's Reptile and Amphibian Medicine and Surgery 3rd (2019).
- Lori Torrini(多个视频)
- Liam Sinclaie(多个视频)
- 排除所有(专职)繁育者自己的“科普”。网上有大量的类似“教程”是繁育者自己写的,循环论证 + 利益相关。应提高警惕。
- 与繁育者的沟通情况
- 沟通本身的质量是极其重要的评价标准。
- 沟通和互动质量如何?
- 沟通态度是否友好、真诚、耐心?(装作小白,观察其是耐心沟通还是爹味十足。)
- 沟通内容是否谨慎、专业、科学?(把自己说成90分的商家可能有80分,但说自己100分(商品绝无问题/知识绝无错误)的商家、多半不到60分。)
- 回答中提供的信息是否全面?
- 是否了解每条蛇的个性?
- 能否出示相关记录?
- 即使繁育者对下述某些问题回答“不知道”、或是答出了你不想听到的答案,单是其真诚、坦率的正面沟通态度就很重要。
- 动物信息
- 物种/亚种
- 选择物种时应考虑物种的特定性情倾向,例如球蟒的性格使其不宜做第一只蛇类宠物。
- 选择物种时应注意法律状态,对在保物种注意买卖和持有相应动物的合法性;如果有法律要求,应索取/办理相关证件。
- 性别
- 出生日期及年龄
- 幼蛇
- 适应新环境的可塑性强
- 可能有未发现的医疗问题
- 喂食状态不稳定
- 亚成体
- 进食稳定,健康史较长,可以通过询问健康记录排除更多医学问题
- 已受后天经历影响。应了解饲养环境、丰容度、上手互动情况和行为训练情况
- 成体
- 健康史更长
- 个性可塑性较低,改变个性更困难;学习行为和适应新事物会花更长时间。必须详细了解生活史
- 有人出售适繁期的成体应格外注重考察是否患病。
- 幼蛇
- 动物捕获/繁殖状态
- 有些蛇如玉米蛇的繁育非常成熟,一般不出现野苗。但购买其他蛇类,如国内有广泛分布的本土蛇,应注意这些问题。
- WC = wild caught 动物直接从野外捡的
- CH = captive hatch 捡的野蛋,拿回来孵化的
- CB = captive breed 自家蛇下的蛋自家孵化。
- 价格 WC << CH << CB,但应该买CB
- 野补几乎100%携病原体
- WC饲养要求高非常多,常出饲养适应问题、喂食问题,圈养压力响应明显(潜伏状态的疾病会在圈养压力下爆发为有症状疾病),动不动死给你看
- CB常更温顺(证据?)
- 避免对野外种群保护造成压力
- 是否自行繁育?
- 事实核查
- 大部分自行繁育者都喜欢发布交配、产蛋、孵化、喂食等日常照片/视频(而且发布日期应符合物种规律)。如果“全是答案、没有过程”,应当警惕。
- 向销售者提出较为广泛的看货要求,要求看多种蛇、或同种蛇的多种变异,并要求提供视频。此时注意观察不同视频中的环境、饲养方法、出场人物等是否统一。
- 自行繁育者可以应需求拍摄新的素材。可提出一些合理但罕见的要求,比如看脖子的腹侧、看泄殖腔、看尾巴尖之类的,这些视频代理很可能没有提前准备。
- 要求视频电话
- 如果卖家售卖的物种范围过于广泛,常是代理。
- 如果是自行繁育、确认:
- 父母的健康状况、年龄、基因状态?
- 父母/祖父母饲养了多长时间?
- 若不是自行繁育(很多是代理):
- 繁育者是谁?
- 你如何进货的?
- 在你这里养了多久?
- 如果已经养了一段时间,为何考虑要买来这条蛇、现在又要卖掉?
- 国内有时兴“中介”的特色现象,这超出了我的理解能力,不做评述。
- 事实核查
- 有些蛇如玉米蛇的繁育非常成熟,一般不出现野苗。但购买其他蛇类,如国内有广泛分布的本土蛇,应注意这些问题。
- 变异及外观
- 核实变异类型
- 网购时,注意了解各种物种的个体识别特征是什么,例如对某些爬行动物是头纹。做选择和最后收货时应时刻注意个体识别特征,避免视频张冠李戴、避免掉包。
- 如果你在意基因质量,注意蛇是不是近亲繁育集齐罕见基因过程中的”下脚料“
- 注意相关变异成体的外观形态。不要仅看幼体的样貌选购,应关注成体花色是否喜欢。可通过morphmarket.com了解,或要求繁育者出示父母的影像(当然父母的外观不一定和孩子一样)
- 该蛇是否曾参与繁殖、是否曾产蛋(两个问题互相独立)
- 销售者是否有其他宠物(尤其是爬行动物)?如果有,具体是什么情况?末次添加新爬行动物的日期是什么时候?隔离措施如何做的?
- 销售者是否与其他爬行动物接触(包括自己宠物、他人宠物、兽医院接触、出门野采等)?
- 物种/亚种
- 性情测试
- 向出售者询问;
- 该个体的个性如何?
- 是否在幼年提供了足够的运动、丰容和探索机会?这将明显影响身体和智力发育。
- 平时是否有接触新事物的训练?
- 平时是是如何上手的?上手有何响应?(恶劣的“漫灌”式上手训练会影响渐近脱敏)
- 它喜欢进行哪些行为?
- 是否为蛇适应新环境做了准备?
- 是否曾有行为训练?
- 平时的互动状态如何?
- 平时喂食和清洁时蛇有什么反应?
- 试验(自己完成、或由销售者代为完成并做视频记录):
- 打开栖地门/拉出抽屉,后撤远离、观察动物行为
- 打开栖地门/拉出抽屉,接近至接触距离,但不碰触
- 向栖地中置入陌生物体(任何安全且蛇未见过的物体均可)
- 考查做出上述操作时是否出现:
- 行为冻结
- 躲避
- 远离-回避行为
- 警戒姿态、喷气、咬击
- 向门移动
- 吐舌、嗅闻
- 探索/走出栖地
- 爬上手臂
- 向新物体移动
- 爬到新物体附近
- 接触新物体
- 其他行为
- 向出售者询问;
- 病史
- 是否定期体检?请出示记录。
- 有无物种特定的常见病原体检查报告?包括唾液、粪检(病毒、细菌、寄生虫)具体应检查什么看物种决定。
- 个体健康状况如何?
- 当前有无主诉症状?持续时间?
- 以往有无健康问题?
- 有无外伤史?手术史?
- 是否曾就诊兽医,原因是什么?
- 过去30天内,是否曾给予治疗,请给出详细信息
- 动物行为最近是否有变化
- 亲属:
- 兄弟姐妹:
- 有无夭折?
- 有无疾病?
- 繁育者是否追踪了(已卖出的)兄弟姐妹的健康状况?
- 父母:
- 基因?
- 健康状况?
- 生育时父母的年龄(是否在最适生育年龄)?
- 祖父母:
- 是否还健在?
- 兄弟姐妹:
- 其他动物有无遗传病或传染病?
- 周边有无在隔离个体?
- 体检
- 如果只能在线交易,索要一段清晰的视频,观察运动与肌肉状况、皮肤状况、口鼻外观和有无异常分泌物。
- 线下自己做体格检查,并考虑聘请兽医进行专业体检
- 根据物种送恰当的病原体检查,尤其是如果你家还有其他爬行动物时更要注意
- 呼吸、心跳、体温、体长/重量
- 整体状况
- 精神上是否警觉
- 肌肉力量:全身有无用不上力的部位。蛇的“核心力量”应该非常强。蛇尾应可灵活的卷在物体上,前2/3呈悬吊状态时,蛇应该可以用“腹肌”用力把头部拉回手上才对。肌肉力量弱通常体现有严重疾病。
- 神经疾病:如果肌肉力量看着不弱,但是感觉动作不协调,头歪、无法稳定姿态、失去翻正反射等。
- 体型:根据出生日期衡量体型发育是否合理,且不应该太胖/太瘦。
- 头部
- 吻部、口腔损伤(提示严重压力)
- 口部分泌物
- 口腔炎
- 呼吸道疾病
- 眼睛
- 脖颈
- 运动正常
- 无多余物
- 皮肤
- 皮肤状态(应当光泽油亮,排除脱水等问题)
- 有无开放伤口或伤疤(野蛇很多有伤)
- 缺鳞、腐鳞、烫伤、蜕皮不净(尤其注意眼鳞卡皮、尾尖卡皮、鼻孔内卡皮)
- 末次蜕皮日期;既往蜕皮日期记录;蜕皮的状态,是否正处于蜕皮期
- 不要忘了看腹面
- 有无体外寄生虫
- 身体
- 触诊一遍,排除多余肿物和脊椎畸形(kink)
- 泄殖孔和尾
- 干净,无粪便
- 关注梗阻、卡蛋、气味腺肿胀等
- 生活史
- 要想获得优质的互动体验,必须注重繁育者的饲养条件
- 这不只是为了追求动物福利
- 个体的健康水平、精神状态和智力发育与饲养条件的质量直接相关
- 从小有丰容的个体,与从小只有厨房纸、从小天天应激刻板擦玻璃、从小用高强度频繁强迫上手“脱敏”的个体状态完全不同。
- 这部分很多问题不必真的提问,看一眼就知道了;如果销售者有视频频道,可以额外获取很多信息。
- 栖地大小(大的栖地对身体发育有正面作用,避免小栖地长期压力造成的健康问题)
- 是否配备攀爬(树栖)、水域(水生)
- 垫材(纸,玉米芯,沙,树皮,土)
- 丰容物(丰容水平将影响智力发育)
- 通风(钻孔,纱网,风扇;栖地/爬房通风不加会增加患病风险)
- 栖地卫生状况
- 多长时间清理一次?
- 清理时用何种方法消毒、用何种消毒液、如何消毒?
- 如去现场/有视频,应观察卫生状况
- 拍摄视频/应邀参观时已经是经过选择性美化的结果,这种情况下还卫生堪忧的话应直接放弃。
- 喂食
- 出示详细喂食和体重记录,优秀的饲主都应有相关记录。
- 喂食种类:
- 小鼠(冷冻/鲜杀/活鼠)
- 大鼠(冷冻/鲜杀/活鼠)
- 鸟(何种,冷冻/鲜杀/活体)
- 鱼(何种,冷冻/鲜杀/活体)
- 是否使用补剂?如何使用的?(考察各种营养素缺乏/过量问题)
- 多长时间喂一次?是否每次间隔都一样?
- 末次喂食时间;体重和喂食记录;现在是否因消化状态不宜运输?
- 每次喂的数量、重量?是否只能接受特定的大小?(有无喂的大了/带毛了就吐食?)
- 食物是如何呈递的(必须留在栖地内/可以镊子喂食/可以目标引导...)?
- 最近三次喂食的情况,应当均可以自主进食化冻鼠,
- 要求出示一次喂食视频(但注意刚喂食不能运输)
- 食物来源,是否饲喂野生动物
- 进食行为是否正常
- 排便排尿状况如何
- 光照与加热
- 如何加热
- 卤灯、碳纤维加热灯、陶瓷灯、加热垫、(水栖物种)水加热器?
- 加热器如何隔热?动物可触碰表面的最高温度?
- 如何控温的?
- 温度
- 温度是如何测量的?
- 日间晒点,日间冷区?
- 夜间晒点(如果有),夜间冷区?
- 是否提供其他光源
- 可见光:型号和照度
- UVB:型号和上次更换日期(决定是否有缺钙造成的发育异常)
- 光源的昼夜周期设置
- 是否有直射阳光
- 每日多长时间
- 阳光摄入过程中有无玻璃或塑料遮挡
- 如何加热
- 水和湿度
- 何种饮水设施:水碗、滴水器、喷淋
- 换水的间隔;滴水器和喷淋的时间
- 水源:自来水、瓶装水、野外水源
- 是否使用水补剂、水处理剂
- 有无泡澡设施
- 是否监测栖地湿度,湿度多高
- 是否观察有饮水行为变化
- 何种饮水设施:水碗、滴水器、喷淋
- 家中是否有人抽烟或香薰
- 家中是否有人使用雾化装置
- 最近三个月对动物环境做了什么改变
- 要想获得优质的互动体验,必须注重繁育者的饲养条件
- 寄送和售后
- 尽可能当面交易
- 以文字方式固定所有的约定
- 如需寄送:
- 采取何种寄送方式?
- 要多长时间?
- 如何包装?
- 采取何种控温措施?
- 寄送时的全路程天气如何?
- 开箱视频要求
- 提前以文字方式固定要求
- 保证连续、不间断、不出框。(一个注意事项是录制时打开飞行模式,防止中途电话导致视频中断。)
- 注意仔细展示蛇的各个部位,包括微小的问题,如鼻孔、泄殖腔、尾部
- 应保存一段时间
- 三包服务
- 包含何种内容,如保证存活、健康(无伤,无脊椎畸形,无多余肿物)、无进食问题等?
- 期限是多长?
- 有无长期技术支持?如何联系?
- 注意很多繁育者的动物福利水平极差,应自己查询更高质量的证据,其提供的饲养方法和医学建议应谨慎参考。
- 可以先装作不懂、问一些你明确知道答案的问题,考察其知识上线在哪儿。
- 繁育者是否叮嘱在出现医疗问题告知繁育者?(关心血系健康的繁育者会希望了解售出个体的健康状况)
- 购买后可否用于繁育?
- 繁育者是否反问饲主
- 高水平(高动物福利)的繁育者常对饲主的饲养条件有要求,在交货前常会审核饲主是否已完全做好准备。繁育者若会反过来考察饲主的饲养水平是加分项。
一定不要以“救助有病动物”或者“让动物脱离极差环境”的心态用买蛇来救助。垃圾繁育者、垃圾宠物店的东西一定不要买。你从那儿买了只会让这些垃圾继续在同样环境里让更多动物遭殃。III
去逛了宠物店、爬展之后(尤其是摸蛇之后)一定要充分清洁,否则可能往家里传递爬行动物传染病。IV(Mader, 2019 ![]() )IV
)IV
如果你了解同一个卖家的其他动物,可以通过你了解的别的物种的情况考察即将购买的新物种的情况。(比如你养过守宫,想买玉米蛇,而店中两种物种都有,可以先去看看同一家店的守宫的状态怎么样。)II
【】【2019医书有一章未整理】【】
(Mader, 2019 ![]() )IV
)IV
伏地魔消毒
【造景缸如何铲屎】 如何铲屎,溜蛇,手电筒 【生态缸】
煮沸法消毒时必须至少保持沸腾1~3分钟。注意有些材料无法煮沸消毒,煮沸杀灭了生物污染物,但会产生化学污染物。
。立即清除废物和被污染的基质,并至少每3-4个月更换所有基质一次。
III 水盆的清洁,Reptifile 如果在此之前被弄脏,则使用如F10SC、Rescue或氯己定等动物安全的消毒剂清洗后再填充。
Bioactive enclosure
Bioactive enclosure只是方便饲主,并不一定能提高动物福利。https://youtu.be/DM4vbvm5j1Y
【消毒】
【】【Snake Discovery有两个视频】【】
- 用肥皂水刷洗是其他消毒方法的第一步,不首先去除多余的有机质会影响消毒剂效果
- 高温
- 水煮:水煮适合消毒土壤
- 喷湿后微波加热(可能是瓦泥小守宫说的)
- 水煮人工合成聚合物材质的装饰物(如假植)常导致其因接近熔点而变形,
- 蒸汽消毒机
- 烘干机用于消毒织物材质的丰容物
- 75% 乙醇/异丙醇水溶液,比如挥发干净后再放蛇,对蛇有毒(毒性多少?)。
- F10SC,对宠物安全(可能是瓦泥小守宫说的)
- 活得生物质不做消毒,定期弃去(如苔藓)
- Loritorrini推荐的对cork bark管状躲避的消毒:如果来源与爬行动物无关,且没有其他爬行动物用过,可以不做消毒。如果要消毒,首先用肥皂水刷洗;然后用accelrated过氧化氢产品浸泡(比如Accel或Rescue品牌)
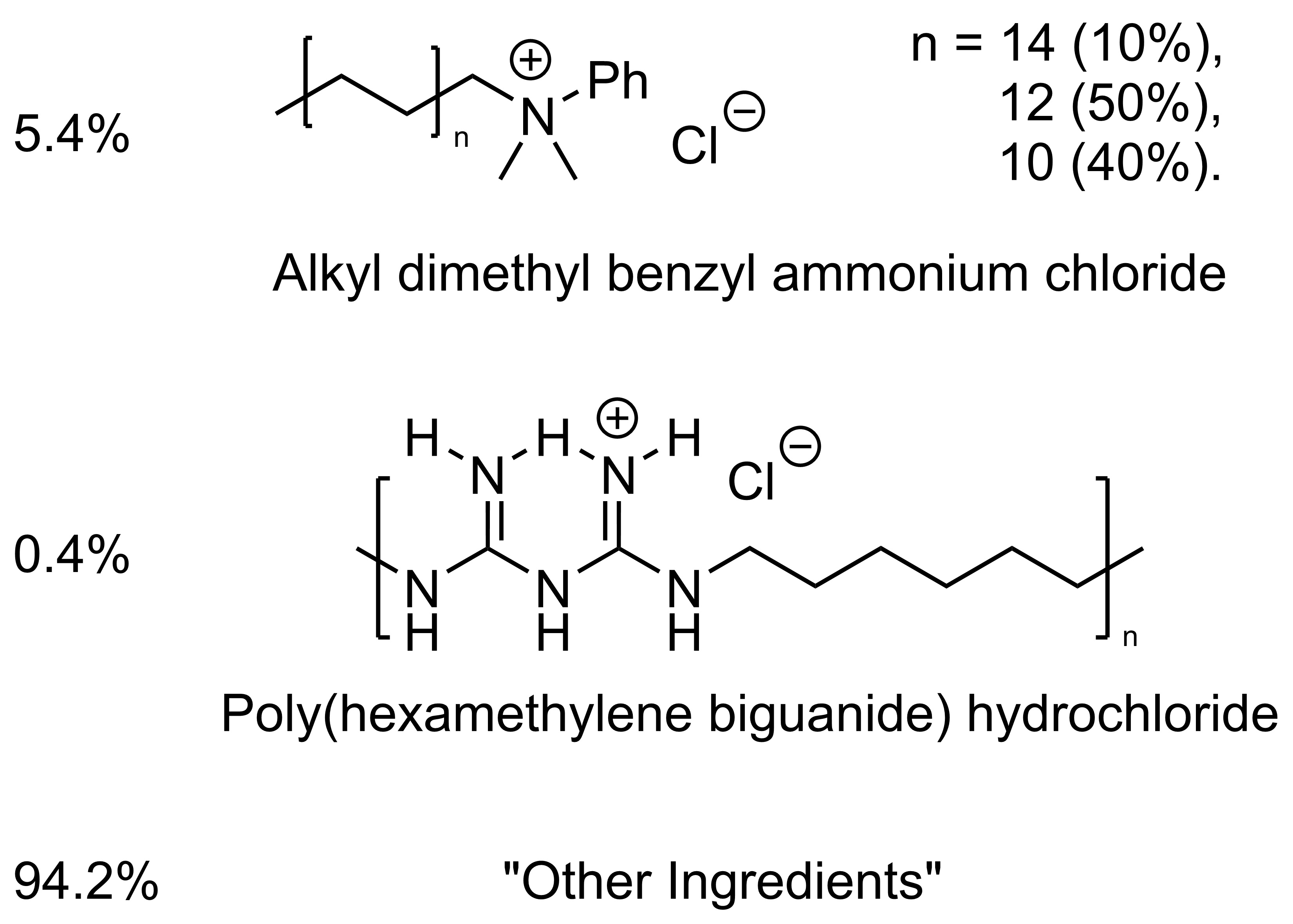
F10SC
Indications: For the treatment and control of upper and lower respiratory tract disease associated with bacterial, fungal, or viral organisms susceptible to benzalkonium chloride and polyhexanide in raptors, pet birds, captive small mammals, and captive reptiles.
For use as a topical antiseptic for surface wounds on raptors, pet birds, captive small mammals, captive reptiles, and captive exotic/zoo mammals.
Use only when there is a reasonable certainty that the treated animal will not be consumed by humans or food-producing animals.
Dosage and Administration: Dilute the concentrate 1:250 with normal saline solution prior to use.
Nebulization:
To Nebulize the glottis, trachea, lungs and air sacs for individual birds, reptiles and small mammals use a chamber connected to a nebulizing unit capable of producing a particle size smaller than 5μm but which allows the resulting “fog” to build-up inside the chamber within 5 minutes and continue for 20 to 40 minutes. Repeat 2 times a day for 2 to 4 weeks (up to 8 weeks in severe cases) until the signs of illness resolve. In raptors, withhold food for 3 hours after nebulization as crop emptying may be delayed. For larger animals or groups of small animals, place them in a suitable sized enclosed room in which a standing fog can be achieved in 5 minutes using a portable electrical atomizer/fogger or a static pressure system capable of producing a particle size of ± 10 to 12μm. Repeat 2 times a day for 2 to 4 weeks (up to 8 weeks in severe cases) until the signs of illness resolve. Nasal and Sinus Flushing:
To remove accumulated mucous and inflammatory material in the upper respiratory tract, syringe into the nasal and sinus cavities (consisting of the external nares, operculum, nasal concha, infraorbital sinus and choanal slit) with the animal’s head down and mouth open to avoid aspiration and allow drainage out of the oral cavity. This treatment should be repeated daily for 10-14 days until the signs of illness resolve. Surface Wounds:
For wound irrigation flush as necessary. For skin decontamination apply as a wash or spray and allow to air dry. Contraindications: Do not use with soaps or other chemicals
Warnings: Not for use in humans. Keep out of reach of children. If accidentally ingested, do not induce vomiting. Give milk or water to drink. If accidental eye contact, hold eye open and rinse with water for 10 minutes. Seek medical help if necessary.
Effectiveness: The active ingredients Benzalkonium chloride and Polyhexanide act on the cell membrane causing it to rupture, resulting in the loss of essential cell components. Additionally, non-toxic ampholytic surfactants and sequesterants in the drug formulation aid in the penetration of the cell or spore wall. Examples of clinical cases successfully treated with F10® brand Antiseptic Solution include upper respiratory tract disease associated with Pseudomonas spp. and Aspergillus spp. in birds, upper respiratory tract disease associated with Pasteurella spp., Pseudomonas spp., Aspergillus spp. and Mycoplasma spp. in small mammals, acute pneumonia and aspiration pneumonia in neonatal mammals, and upper and lower respiratory tract disease associated with Proteus mirabillis, Pseudomonas aeruginosa, Aeromonas spp. and Mycoplasma spp. in reptiles. All resulted in positive outcomes.
Wikipedia 对F10SC的PHMB组分
Products containing PHMB are used for inter-operative irrigation, pre- and post-surgery skin and mucous membrane disinfection, post-operative dressings, surgical and non-surgical wound dressings, surgical bath/hydrotherapy, chronic wounds like diabetic foot ulcer and burn wound management, routine antisepsis during minor incisions, catheterization, first aid, surface disinfection, and linen disinfection.[5] PHMB eye drops have been used as a treatment for eyes affected by Acanthamoeba keratitis.[6]
It is sold as a swimming pool and spa disinfectant in place of chlorine or bromine based products under the name Baquacil.
PHMB is also used as an ingredient in some contact lens cleaning products, cosmetics, personal deodorants and some veterinary products. It is also used to treat clothing (Purista), purportedly to prevent the development of unpleasant odors.
The PHMB hydrochloride salt (solution) is used in the majority of formulations.
Safety Edit In 2011, Polyhexamethylenbiguanide was classified as category 2 carcinogen by the European Chemical Agency, but it is still allowed in cosmetics in small quantities if exposure by inhalation is impossible.
In 2018, the European Commission decided to ban the use of PHMB as a preservative[citation needed]. It is currently used as an algaecide and disinfectant.
第二个成分是氯己定的类似物 Veterinary medicine In animals, chlorhexidine is used for topical disinfection of wounds,[47] and to manage skin infections.[48] Chlorhexidine-based disinfectant products are used in the dairy farming industry.[49]
Post-surgical respiratory problems have been associated with the use of chlorhexidine products in cats.[50]
土壤的消毒
丰容物的消毒
冯柚子的消毒剂介绍
https://www.bilibili.com/video/BV1M84y1t7tB/
(Mader, 2019 ![]() )IV ,(Sinclair, 2020
)IV ,(Sinclair, 2020 ![]() )III
)III
如果你家已经有爬行动物,买新的蛇必须隔离,生有传染病的蛇也要立即隔离。
家里已有其他爬行动物时,新蛇必须隔离后再加入已有种群。新旧蛇的距离越远越好,最好不在一个屋。隔离期间,应该观察新蛇的行为、蜕皮、喂食和粪便情况、和一般健康状况。在照料蛇的时候必须分隔所有的器具。先搞旧蛇,再弄新蛇。搞完新蛇后注意清洁消毒所有沾染物,包括你自己、你的衣服、蛇的器具如蛇勾、喂食夹、清洁工具等等(当然更理想的情况是直接分开两个人来照顾)。
隔离被定义为新动物到达一个群体后,被隔离进行观察、适应新环境、疾病测试以及如有需要的后续治疗或迁移的一段时间。无意中将传染性病原体引入已建立的爬行动物群体,一死一大批,可能会导致情感和经济的双重打击。
隔离的作用既有保护既有种群,也为新蛇提供缓冲时间,减少压力,有充分的证据表明,运输和暴露于新环境可能会带来压力,可能使动物更容易感染并更有可能排出传染性病原体(参见压力一章)。
亚特兰大动物园规定未知来源的爬行动物至少隔离90天。这样才能度过大多数病原体的潜伏期。Sinclair认为最好有六个月。
如果爬行动物来源已知(且可信),并且有详细的健康记录,在评估期间(disease risk assessment)未发现潜在有害因素,则隔离可以调整至14天。
新蛇的隔离栖地应该平衡容易清洁的程度和丰容程度,使得既不太增加彻底情节的难度,又要通过恰当丰容减少给蛇的压力(尤其考虑到蛇的迁移本来就是很大的压力事件)。
隔离栖地的垫材目前认为直接用纸是较好的选择。一是便宜、容易更换,二是因为它白、且不会污染样品,所以便于收集粪便和尿酸做寄生虫等检查。
虽然隔离期间的福利可以适当的为易清洁性做出牺牲,但因为隔离期并不短,不应该为了隔离而长期的压制动物需求,很多丰容物和栖地布置有更易情节的版本,如攀爬物可从自然且有褶皱的软木树枝换成塑料儿童玩具等
隔离动物应该有详细的记录,包括体态、体重、食欲、行为、排便、粪便外观等,注意有无吐食、腹泻、拒食、体重减轻、蜕皮异常、乏力不动、移动异常、脱水。
隔离期间发现问题就应该延长隔离,并尽快诊断筛查病因。
要严格防止隐孢子虫病(cryptosporidiosis,常缩略为crypto), intranuclear coccidiosis(核内球虫病)和变形虫症/阿米巴症(amoebiasis)。隐孢子虫和核内球虫的检查金标准是PCR监测,北京上海各有一些地方能做;变形虫可以用粪便染色的方法做。
血清学检查作用有限,可以用于给蛇检查副黏液病毒病(ophidian paramyxovirus);阳性有病,阴性不一定没病。
注意外部寄生虫,如蛇螨(Ophionyssus natricis),应该反复多次检查有无外部寄生虫(有时寄生虫夹在鳞片之间看不到,需要等待他们运动到外面的时候才能看到),如果是野苗,要格外注意注意蜱虫。
其他要主义的疾病有Herpesviruses, Sunshinevirus, Ferlavirus 和 adenovirus,如果你的已有种群足够大、足够珍贵,应该一定注意花点钱做筛查。
如果同时有多只动物在隔离期内,最好采取全进全出(all in/all out)的策略,根据最后到达的动物计算隔离期,不要像方舱似的搞轮换,后者非常可笑。
注意,和其他爬行动物接触的人也具有传染爬行动物疾病的风险,比如爬友互相串门互相上手可能导致疾病传播,逛爬展、逛宠物店更是重要传播途径,应该格外注意这些时候的隔离消毒工作,最好在这些活动之后换洗衣服。
注意和其他爬行动物接触的器具也是传染源,比如一家店同时卖动物和动物用品(比如垫材),那他们的垫材拿回来也应该隔离或者通过煮沸等方式彻底消毒,否则可能传染蛇螨等疾病。
各种疾病列表: 【】【 https://www.merckvetmanual.com/exotic-and-laboratory-animals/reptiles/bacterial-diseases-of-reptiles?ruleredirectid=400 https://reptilesmagazine.com/common-reptile-viral-diseases/
Signs of Illness and Stress in Reptiles https://www.anapsid.org/signs.html】【】
(Mader, 2019 ![]() )IV
)IV
每次上手时的体格检查
鼻子嘴的状态
呼吸声
鳞的状态
vent的状态
屎的状态(屎异常时注意收集)
称重的频率
体重降低很危险
蜕皮后的检查
鼻子卡皮
眼鳞卡皮
尾巴尖
每三周掰开口腔看看
Snake Discovery有一个视频
ReptiFiles
- silver ointment
- 消毒剂(Betadine, povidone iodine)
- chlorhexidine solution (ex: Nolvasan)
- 纱布
- 用于固定纱布的Vetrap弹性包扎带
- 小型防水创可贴
- 非杀精的避孕套,用于包扎蛇和蜥蜴的尾巴
- 一次性压舌板
- 棉签:消毒、喂药、用棉签棒掰开嘴
- 镊子
- 用于寻找螨虫的放大镜
- Nix lice treatment,用于螨虫和对新爬行动物的预防性治疗
- 无味Pedialyte,用于给脱水的爬行动物补水
- NutriBAC或Bene-Bac Plus益生菌补充剂,用于从驱虫药和抗生素中恢复
- EmerAid Omnivore and the EmerAid Critical Care System 肉食配方
- 注射器
- 厨房秤,用于监控体重
- 常规兽医和带急诊动物医院的电话号码
- 请务必检查任何药品的有效期,并根据需要进行更换。
带好记录
收集排泄物
呼吸道、皮肤、肠胃
国内有许多人(包括部分宠物医生)认为国内的蛇很少有寄生虫病,有时即使出现了特征症状,也对寄生虫病的可能不屑一顾。实际上国内宠物市场的寄生虫病并不罕见,尤其是看到售卖亚成体和成体时候一定要考虑是不是有难以医治的寄生虫病。要了解寄生虫病的症状特征,比如看到“两岁亚成体,母,吐过一次调理好了,短期内只能吃无毛乳鼠”就要高度怀疑是不是隐孢子虫感染。家里已经有爬行动物买蛇时,新蛇到家一定要隔离,非常推荐做一套常见寄生虫检查。(那个冯柚子隐孢子虫视频 ![]() )
)
https://www.bilibili.com/video/BV1dT411y7CP/
https://www.bilibili.com/video/BV1Vg4y1575n/
https://www.bilibili.com/video/BV1Gj411e7uQ/
| 拉丁 | 德语 | 英语 | 中文 |
|---|---|---|---|
| Oxyuriden | Oxyuridae | 尖尾线虫 | |
| Ascariden | Ascarids | 蛔虫 | |
| Heterakiden | Heterakids | ||
| Rhabdias | Rhabdias | 桿线虫属 | |
| Strongyloides | Strongyloides | 类圆虫属 | |
| Spiruriden | Spirurids | 司比路目 | |
| Zestoden | Cestodes | 绦虫 | |
| Trematoden | Trematoda | 吸虫 | |
| Pentastomiden | Pentastomids | 五口虫/舌形虫 | |
| Trichomonaden | Trichomonads | 滴虫 | |
| Kokzidien | Coccidia | 球虫亚科 | |
| Isospora | Isospora | 球虫属 | |
| (Choleo-)eimeria | (Choleo)eimeria | 艾美球虫 | |
| Nyctotherus | Nyctotherus | 夜出纤毛虫属 | |
| Balantidium | Balantidium | 小袋虫属 | |
| Amöben | Amoeba | 变形虫 | |
| Hexamiten | Hexamita | 六鞭毛虫属 | |
| Cryptosporidien | Cryptosporidium | 隐孢子虫 | |
| Darmflagellaten | Darm-flagellaten | 肠鞭毛虫 | |
| flagellaten | 鞭毛虫 | ||
| Darmziliaten | 肠纤毛虫 | ||
| ziliaten | 纤毛虫 | ||
| Agamen | Agamids | ||
| Leguane | Iguanas | ||
| Warane | monitor lizard | ||
| Pfiriemenschwänze | Pinworm | 蛲虫 | |
| Madenwurm | Pinworm | 蛲虫 |
- Ascarids 蛔虫
- Hexamites 六鞭毛虫属
- Coccidia 球虫亚科
- Trematodes 吸虫
- Cryptosporidia 隐孢子虫
- Darm-flagellaten 肠鞭毛虫
- Darm-ziliaten 纤毛虫
- Trichuris muris 鼠鞭虫:2%
- Protozoa 原生动物
- Sarcodina 伪足虫纲 (amebas 变形虫)
- Sporozoa 孢子纲 (sporozoans 孢子虫)
- Mastigophora 鞭毛虫纲 (flagellates 鞭毛类)
- Ciliata 纤毛纲 (ciliates 纤毛虫)
- Metazoa 后生动物 (helminths 蠕虫)
- Platyhelminthes 扁形动物门 (flatworms 扁虫)
- Trematoda 吸虫纲 (flukes 吸虫类)
- Cestoda 绦虫类 (tapeworms 绦虫)
- Nemathelminthes 圆虫动物门 (roundworms 蛔虫)
- Platyhelminthes 扁形动物门 (flatworms 扁虫)
寄生虫的定义:寄生方利用宿主获益,并使宿主受到伤害
以下参考:
(Miranda 2024, 2024 ![]() )III
)III
Paula Sapion Miranda, 墨西哥人,于柏林自由大学获得了我的兽医执照,在兽医实验室exomed GmbH工作。贾斯图斯-李比希大学兽医寄生虫学博士在读,研究“爬行动物和两栖动物的内寄生虫”。
并不是所有的寄生虫在不同动物中的感染频率都一样
圈养蜥蜴中内寄生虫的出现频率(中文翻译不一定准确)
| Agamids | Iguanas | Chameleons | Geckos | Monitor Lizards | |
|---|---|---|---|---|---|
| 数量 | 386 | 164 | 66 | 68 | 21 |
| 阴性率 | 16% | 29% | 49% | 26% | 28% |
| Oxyuridae 尖尾线虫 | 61.6% | 43.2% | 27.3% | 51.5% | 4.7% |
| Ascarids 蛔虫 / Heterakids | 1.5% | 0.6% | 6.0% | 2.9% | - |
| Rhabdias 桿线虫属 / Strongyloides 类圆虫属 | 1.3% | - | 1.5% | 2.9% | 14.3% |
| Spirurids 司比路目 | 0.5% | 1.2% | 1.5% | 1.5% | - |
| Cestodes 绦虫 | 0.3% | 11.6% | - | 2.9% | 19.0% |
| Trematodes 吸虫 | 0.3% | - | 4.5% | - | - |
| Pentastomids 五口虫/舌形虫 | - | - | - | 5.8% | 4.7% |
| Trichomonads 滴虫 | 11.6% | 20.2% | 13.6% | 14.7% | 28.6% |
| Isospora 球虫 | 43.5% | 0.6% | 7.6% | - | - |
| (Choleo)eimeria 艾美球虫 | 1.5% | 1.8% | 3.0% | 10.3% | 4.7% |
| Nyctotherus 夜出纤毛虫属 | 13.9% | 3.0% | 1.5% | 14.7% | - |
| Balantidium 小袋虫属 | - | 5.5% | - | 1.5% | - |
| Amoeba 变形虫 | 1.0% | 20.7% | - | -% | - |
- 寄生虫
- Protozoa 单细胞的,原生动物
- Sarcodina 伪足虫纲 (amebas 变形虫)
- Sporozoa 孢子纲 (sporozoans 孢子虫)
- Mastigophora 鞭毛虫纲 (flagellates 鞭毛类)
- Ciliata 纤毛纲 (ciliates 纤毛虫)
- Metazoa 多细胞的,后生动物 (helminths 蠕虫)
- Platyhelminthes 扁形动物门 (flatworms 扁虫)
- Trematoda 吸虫纲 (flukes 吸虫类)
- Cestoda 绦虫类 (tapeworms 绦虫)
- Nemathelminthes 圆虫动物门 (roundworms 蛔虫)
- Platyhelminthes 扁形动物门 (flatworms 扁虫)
- Protozoa 单细胞的,原生动物
线虫 nematodes 是两爬最常见的寄生虫,主要存在于肠道
- Ectoparasites 外寄生虫
- 生活在体表的寄生虫,比如虱、螨、跳蚤
- Endoparasites 内寄生虫
- 通常用肉眼看不见,尤其是中间阶段,都要用显微镜
- 不同寄生虫的外观差别很大
在野外,宿主动物和寄生虫之间维持平衡;在圈养压力下,平衡被打破,寄生虫的负面影响变大,易感性增加。
寄生虫病的症状常不特异,如拒食、消瘦等,不诊断难以用药
辅助寄生虫感染的控制、预防
自然界中动物活动范围大,重复感染罕见;野生动物载量低,症状轻
圈养动物活动范围小,重复感染几率大;有的卫生条件恶劣,动物密集,增加积累大量寄生虫的风险,可能导致严重问题
预防人畜共患病,因为75%的人类疾病都是人畜共患病。诊断可以用于发现新发待关注疾病
粪检方法
-
Native "Direct saline fecal smear" 原生盐水粪便图片
- 最快,5秒就完成制样,只需加一点染料和(滑石粉?),载玻片上滴一滴,盖上盖玻片,观察即可
- 可以检测 protozoa 原生动物和 metazoa 后生动物
- 灵敏度可能较低,一些原生动物不容易被浮选出来
-
沉淀/浮选法
- 测试稍复杂
- 灵敏度较高,能看到原生涂片看不到的东西;但也有研究说三种方法的灵敏度没有显著性差异
- 运动性差的原生动物检出能力较低,因为不易上浮
-
SAF (sodium acetate - formalin 乙酸钠/甲醛溶液法)
- 冻结寄生虫德演化周期
- 需要溶液制备,试剂有毒,家里不能用,测试更繁琐
- 不再能检测到活动性的原生动物
- 可以用于要长期保存的取样,如野采
- 有健康风险
- 干扰宿主营养吸收
- 破坏器官,导致器官衰竭,如鼠螨属寄生虫
- 必须控制寄生虫数量,避免过度繁殖
- 控制寄生虫提升宿主福利
- 避免继发感染
- 控制动物间传播和动物到人的传播
寄生虫并不是都致病
- 绝对致病 obligate pathogen
- Ascarids 蛔虫
- Hexamites 六鞭毛虫属
- Coccidia 球虫亚科
- Trematodes 吸虫
- Cryptosporidia 隐孢子虫
- 条件致病 facultative pathogen,是肠道微生物群落的一部分,不超额繁殖就没问题
- Darm-flagellaten 肠鞭毛虫
- Darm-ziliaten 纤毛虫
意大利蛇类中寄生性和伪寄生性病原体的流行率 (Rinaldi 2012):
- 伪寄生虫(其实比真寄生虫还多,是“肠道过客”,不给宿主带来健康风险,导致假阳性,不要看见寄生虫就盲目给药)
- Syphacia obvelata:0.8%
- Trichuris muris 鼠鞭虫:2%
- Hymenolepis nana (Dwarf tapeworm):2%
- Aspiculuris tetraptera 鼠蛲虫?:10%
- Myocoptes musculinus:36%
- (真)寄生虫:
- Pulmonary nematodes 肺线虫:1%
- Other gastrointestinal nematodes 其他胃肠道线虫:2%
- Strongyloides spp.:2%
- Capillaria spp.:2%
- Emeriidae:10%
意大利蜥蜴中的伪寄生虫和真寄生虫的流行率 (Rinaldi 2012):
- 伪寄生虫:
- 螨:12%
- (真)寄生虫:
- Emeriidae:36%
- Oxyurids:48%
常见的伪寄生虫
- 食肉动物中的伪寄生虫:
Myocoptes musculinus(鼠螨?Mausenmilbe),图中主体是卵,上面是毛
Hymenolepis nana (Dwarf tapeworm)
Syphacia muris 一种鼠类的线虫,不应误诊为蛇自己的寄生虫
Aspiculuris tetraptera 一种鼠类的线虫,不应误诊为蛇自己的寄生虫 (鼠蛲虫?)
- 食草动物中的伪寄生虫
Monocystis spp. 的一个Gemontenziste,通常在Schilgroten和Exen的粪便中发现,但实际上是Rogenbohr-Protosin类型的寄生虫?
Adelina spp. 的卵囊,一种昆虫寄生虫,全世界都有分布,有时在猴子的粪便里能找到
植物花粉
部分寄生虫可能造成冬眠期间的死亡
冬眠前后要检查并治疗寄生虫,治疗应在冬眠前6周开始,避免携带药物进入冬眠
英文 pinworm
德文 Pfriemenschwänze (owl tail)
- 动物界 Animalia
- 线虫动物门 Nematoda
- 色矛纲 Chromadorea
- 小杆目 Rhabditida
- 尖尾线虫科 Oxyuridae
- 蛲虫属 Enterobius
- 蛲虫 pinworm 是食草和食虫动物中分布最广泛线虫。
- 蛲虫生活在大肠中。
- 由于其生命周期直接、快速(最长不超过40天)。如果不清理粪便,很快就会积累很高的虫载量。
- 圈养个体的高载量感染可能导致乏力、体重减轻、拒食、腹泻、呼吸困难、泄殖腔或阴茎脱垂、生长和繁殖障碍以及冬眠期死亡。
- 爬箱清洁(尤其是及时除去粪便)、病原体治疗和治疗次生疾病同等重要。不充分清洁栖地光治动物是没用的
- 卵壳厚,耐受性强
生命周期:吃入卵,孵化为幼虫在肠道中生活,成虫排卵,随粪便排出
蛲虫重度感染 -> 虫在盲肠团块 -> 导致便秘 -> 可能会导致肠梗阻
冬眠后可能复发
下图可见整个肠道肿胀,左下切开的肠道可见虫体
膨胀的肠体可能压迫肺部导致呼吸困难
幼年动物,蛲虫吸收营养可能造成营养不良,可能增加肾病综合征、肾炎、代谢骨病的发生率,蛲虫会损坏肝肾,肾脏功能受损后影响D3生化,导致代谢骨病,进而导致龟壳的软化 (Hallinger 2018)
下图是感染造成的次生影响
下图是软化的龟壳
下图是损坏的肝
拉丁文: Rhabdias/Entomelas spp. (Nematoda)
- 杆线虫感染发生于水生生物或两栖类中
- 影响食肉/食虫生物
- 高感染率,高重症率;严重程度有差异,从无明显临床症状到危及生命。
- 卵壳薄,卵中含rhabditiform L1时期的幼虫(杆状幼虫)。
- 从通过气管感染进入消化道。
- 这些线虫在德国饲养的anurans中广泛存在,感染率为23%(Hallinger 2020)
生命周期,L1阶段的幼虫从气管进入,进入呼吸道,卵排出体外
图 (Gendron 2003)
主要寄生在Nattern, Bipan Axten, Agaments的动物,以及Kings和变色龙上
并不像Anupen一样频繁出现,但是还是能见到
Ja das Interessante sind, dass Adulter Reptiles immer Froditen sind, also im Endellunge die Produzien spermieren und dann machen die die in einer Samentasche und dann Produzien, die auch noch Huvahren nutzisten und die vermehren sich dann da fort, polizierende Eier und wenn die Eier ausgeschehen werden, dann gibt es zwei verschiedene Larven, also ein paar Larven werden zur L3-infektioese Larven und werden wieder aufgenommen, Moral oder Amphibien, da kommt die auch Subkotan rein und wandern durch ein Blutsystem und Lymphsystem in der Lunge, aber die anderen Larven, also die können sich auch getrenntgeschlechtlich vermehren und da auch Eier freisetzen im Terrarium, also es geht, wir haben jetzt zwei Methoden entwickelt, um sich fortzuflenzen und hier haben wir ein Video von letzte Woche, das war im Baumsteiger, frisch wenn ich mich nicht irre, da sind wir auch ein paar Zillaten, die sind aber eher nicht das Problem, da werde ich aber auch was dazu sagen.
有趣的是,这种成年的Reptiles总是雄性的。在它们成熟的最后阶段会产生精子,然后他们将精子放入一个精囊中,然后利用已经产生的Huvahren进行繁殖。他们会产生多孔的卵,当这些卵被排出之后,会生成两种不同的幼虫。一些幼虫会成为感染性的L3幼虫,然后会被吸收进入植物或者两栖动物的体内。它们会从皮下进入,通过血系统和淋巴系统在肺部游动。但是另一些幼虫,它们能够通过异性繁殖方式释放出更多的卵在饲养环境中,也就是说,我们有了两种方法来进行繁殖。在这里,我们有一段上周的视频,我如果没有记错的话,这是在树上拍摄的,我们也看到了一些Zillaten,并不是大问题,我会对此进行一些说明。
【】【有视频】【】
Kalicephalus sp.
- 爬行动物中重要的致病感染。
- 主要发生在蛇类中。
- 生活在肠道中,损害黏膜。导致吸收障碍和失血。
- 乏力、拒食和消瘦是线虫感染的主要症状,症状不特异。
- 圈养中的病原体密度科迅速增加
- 经常伴随有继发性感染(沙门氏菌 Salmonella spp.、气单胞菌 Aeromonas、假单胞菌 Pseudomonas)。
钩口线虫科 Ancylostomatidae 的头部 (Kalicephalus sp.): 注意到面颊的空腔结构帮助在肠黏膜上的固定 Hallinger et al. (2019)
Kalicephalus 属生物的卵,薄壁,但内无幼虫,先和Plastomere一起排出,然后才发育为幼虫:
尸体解剖,气味难闻,肠壁会肥大,观察到对一侧的侵蚀
- 动物界 Animalia
- 线虫动物门 Nematoda
- 色矛纲 Chromadorea
- 小杆目 Rhabditida
- 旋尾亚目 Spirurina
- 蛔形下目 Ascaridomorpha
- 蛔虫总科 Ascaridoidea
- 蛔虫科 Ascarididae
- Hexametra spp
蛔虫(线虫门)
少见但危害大
- 在爬行动物中引起重要的致病感染。
- 蠕虫生活在胃肠道中,吸附于粘膜,甚至穿透粘膜。
- 在龟中是直接生命周期 Direct cycle
- 在蛇中是间接生命周期 Indirect cycle 伴随体内迁移
- 在蜥蜴中为partly?
- 两栖动物/啮齿动物作为中间宿主(可能在变色龙中为昆虫作为中间宿主)。
- 消瘦、乏力、食欲不振是蛔虫感染的主要迹象。
- 可能由于胃肠穿孔猝死,或在体内迁移过程中因损伤中央血管导致猝死。
Reitl 2020
生命周期:
解剖显示皮下布满虫的成体。该动物治疗后仍然死亡 Reitl 2019
赫曼陆龟中蛔虫的繁殖无中间宿主,增加了危险性
赫曼陆龟样品中的蛔虫感染 下图中像眼睛一样得椭圆形是蛔虫卵,靠近最中间一个蛋型的是Ascaridae,因为向下凹陷,不规整,较厚
赫曼陆龟的蛔虫感染视频,有一条完整的虫,10cm长
【】【】【】
鳄鱼也可以感染,但是鳄鱼体内的虫只有2 cm,在变色龙里的虫可以长到17 cm,可以穿透一切东西
高冠变色龙蛔虫感染后皮下组织中的幼虫,有Ascarids的幼虫,皮下有结节,切开结节就是虫。右上角是幼虫的嘴
另一类寄生虫
Saugwürmer,flukes(Trematoda, Digenea)
Spirometra sp.
圈养动物罕见,多是野捕或生活在水边的动物才有;
本处讨论Degenantramatoden
- 动物界 Animalia
- 扁形动物门 Platyhelminthes
- 被杆体亚门 Rhabditophora
- 无中心体类 Acentrosomata
- 裂肠新皮类 Bothrioneodermata
- 新皮总纲 Neodermata
- 吸虫纲 Trematoda
这是带卵壳的蛋,是Trematoda的典型特点
Ein typischer Eier mit Deckel, also hier kann man auch sehen, das ist so auch ganz typisch für Trimadodeneier. 一个典型的带盖子的蛋,这里你也可以看到,这也是Trimadodene的典型特点。
Genau, bestimmte Saugwürmer bei Simpschilkröten haben einen direkten Entwicklungsschüttlosverlauf, also das sind die monogene Haken-Saugwürmer, die gehören also nicht zu diginär, aber das sind auch saugwürmer an sich und die erinnert man aber auch oft bei Fische wie die Ikeemwürmer und die Hautwürmer, also da Kirodaktelus vielleicht hat der eine oder andere auch davon was gehört. 的确,在Simpschilkröten中,有一些特殊的吸虫具有直接的发育过程。这些单基因的钩吸虫并不属于diginär类别,但依然是吸虫。对于喜欢研究鱼类的人来说,Ikeemwürmer和Hautwürmer可能并不陌生,可能有些人已经听过关于Kirodaktelus的一些事情。 Ja, die kommen auch bei Simpschilkröten vorkommen. 就是说,这种吸虫也会在Simpschilkröten中出现。
- 绝对致病病原体,大多数为内寄生虫
- Spirometra sp.常有两个吸盘(口和腹部)
- 性繁殖伴随有性繁殖和宿主变更
- 两栖动物和爬行动物可以充当中间宿主或最终宿主
- 致病性通常较低
- 传播率低,生命周期通常不能在爬箱里完成,圈养动物罕见,在户外养殖(例如,池塘系统)或不卫生的养殖条件下可能发生
- 生命周期需要1~2个中间饲主,其中至少有一个要是蜗牛或软体动物,所以圈养中少见
短角变色龙(Elefantenohrchamaleon)中的吸虫及其卵(黑点点),旁边就是两个吸盘(中央和右侧的大黑圆)。作者:Biallas (2014)
在一只青蛙(O. lima)的肠道中的 Diplodiscus sp. 作者:Hallinger (2019)"青蛙体内的Trimadoten 青蛙身上的Trimadoden很少是成体,大部分都是中期寄生虫
Hier ist ein Trimadoten von Frosch. 这就是在青蛙体内找到的Trimadoten。
Man findet ganz viele Zwischenwürtt-Stadien, also die Metazerkarien bei den Fröschen, weil die dienen sozusagen als Nahrung für den Entwürtt. 在我们进行的研究中,发现了大量的中间生长阶段的昆虫,比如青蛙身上的Metazerkarien,因为它们基本上是作为Entwürtt的食物来源。
Belegentlich werden auch Trimadoden in der Mundhöhle von Wildgefangenen, Schlangenfunden, die heißen auch Soma, aber die habe ich auch noch nicht selbst gesehen, so live. 在一些野生捕获的蛇的口腔中,我们也会偶尔发现Trimadoden,这些也被称为Soma,但我并没有亲自看到过。
【】【35:09】【】
【】【Bogan2019, cryptosporidium in snakes a review.pdf】【】
隐孢子虫即Cryptosporidiosis,英文语境下常被缩写为Crypto
https://www.bilibili.com/video/BV1RG411p7gf/
非常危险,死亡率高,传染性强。
可通过抗酸染色或PCR方法来检测。尤其注意新蛇是否有此问题。
荧光定量PCR检测可以邮购检测设备,在干净的垫材上(如宠物尿布)收集新鲜的粪便送检。
隐孢子虫可能导致腹部“胀气”,实际是腹水(http://b23.tv/EPi5aJp),可能导致频繁吐食,或者可能有拒食、腹泻。出现这些情况的蛇预后差,死亡率高。
蛇类感染隐孢子虫无法自愈、无药物可以根治(http://b23.tv/EPi5aJp)。
隐孢子虫的无症状感染者会持续排出寄生虫卵囊,持续威胁其他健康蛇(http://b23.tv/EPi5aJp)。
【】【自己视频中的内容未整理】【】
文献中用于器具表面的(不能用于动物)对Cryptosporidium parvum的卵囊的有效消毒剂有:
8%的氨水24小时可以杀灭到千分之一以下;
6%的过氧化氢作用20分钟可以杀灭到千分之一以下,作用13分钟时还不行;
湿热法60°C45°C,59 min,“有效杀灭”
环氧乙烷蒸汽可以杀灭到千分之一以下
不能有效杀灭的杀毒剂: 3% 过氧化氢,2.5%戊二醛,0.35%过氧乙酸,70%乙醇,70%异丙醇,1% 聚维酮碘,1%苯酚,5% 次氯酸钠,1% 季铵盐,邻苯二醛。
Mader的兽医书中说类似的消毒效果报道大部分是针对Cryptosporidium parvum,不是两爬致病种,应该谨慎考虑是否有种间差异。
Cryptosporidium parvum(鼠隐孢子虫)在热水中 73度1分钟,64度2分钟就可以灭活,即用煮完后的水给老鼠接种,未发现老鼠感染寄生虫(10.1128/aem.60.8.2732-2735.1994)
紫外线:文章 10.1016/j.pt.2004.11.009 总结了很多文献的结果,需要大约9~10 mJ/cm^2 的254 nm紫外线才能做到三个数量级的杀灭效果,另外应该考虑紫外线杀菌无法拐弯的通病,需要表面平整(比如适用于操作台面的灭菌、不太适用于许多容器的灭菌);另外UVC灭菌操作对人员的操作安全水平和操作环境有一定要求。
冷冻可以杀死隐孢子虫吗:H. V. Smith在1992年测试了C. parvum的环境抗性。结论是在-22°C下冷冻卵囊21小时后尚余33%存活,冷冻一周后还有10%存活,冷冻31天后还有1.8%存活。作者还得出液氮速冻完全杀死,直接暴露在气流风干造就的干燥环境中可完全杀死,粪便样品在48天后仍有显著比例存活。(Appl. Environ. Microbiol. 1992, 3494)。G. Duffy 从食品安全角度测试了 C. parvum 卵囊残留在牛肉中时对冷冻的抗性,经过速冻(60 h内降到-20 °C)、保存(-20 °C保持 3 周)、化冻的周期后,还有7~10%的卵囊存活。(Meat Sci. 2004, 67, 559)
当然还要排除是不是别的隐孢子虫,也有可能是老鼠携带的并不制病。食鼠的爬宠粪检检出对爬行动物不致病的 C. muris 和 C. parvum 概率很高。Yimming 等人检测 165 份份粪便样品中有12份抗酸染色阳性,40份PCR阳性,其中27份是 C. parvum或C. muris,只有9份是致病的C. serpentis(Korean J Parasitol. 2016, 54, 423)。不同的隐孢子虫可以用PCR区分。不能仅依据镜下粪检就做出处死的决定,应该结合活检和病理确认有发病后再做决定。
上海有家大宠物医院说是开发了PCR定种的商业医疗检测方案。上海绮异异宠医院,我加的成奇医生的微信,看见他朋友圈里发本院提供区分蛇,蜥蜴隐孢子虫的检测项目
Mader的两爬内外科学教材也提到了硝唑尼特,将其与巴龙霉素并列为被使用但无法可靠清除感染的药物(“There are no known effective treatments for cryptosporidiosis in reptiles. Drugs such as paromomycin or nitazoxanide have been used and do not consistently clear infection.”)。
III
【】【Snake Discovery有一个视频】【】
寄生虫疾病。虫很小但肉眼可见。生存方式是吸食爬行动物的血液。
【预防】 一般性传染病预防:控制传染源,切断传播途径,保护易感蛇群。
- 避免接触感染动物,如宠物店、爬展、野蛇(野蛇的蛇螨感染极为普遍)。
- 接触(除自家动物之外的)其他爬行动物之后充分清洁。
- 避免物品传播:
- 从爬宠店购买的用品要用次氯酸钙等消毒剂处理。最好避免从有动物的爬宠店买东西。
- 垫材最好不要从有动物的爬宠店买(最好直接从和爬宠没有任何关系的地方买,比如可靠的花店,不过要自己核查垫材的种类是不是适合爬宠)
- 垫材最好经过隔离措施,比如在家放置2个月再用;也可以走高温消毒流程,比如放锅里用开水煮一遍(然后放凉,用手挤掉多余水后使用)也可以喷湿之后放微波炉加热。
【症状】
- 瘙痒:蛇反复蹭东西蹭地板
- 乏力、对事物兴趣减退或拒食
- 泡澡(泡澡可能有其他原因,也可能是正常的,注意甄别)
- 能肉眼看到螨虫,主要集中于眼镜、耳朵、泄殖孔、和褪下来的皮上;上手之后也可能跑到你手上。尺寸很小,有的时候像是皮肤上的斑点;和斑点区分除了用显微镜之外;还可以注意斑点是不是会移动。
- 能看到螨虫的粪便,体现在鳞片上看着像有灰尘。
【病因】 接触传染源。
【治疗】 Reptifile: 推荐用Nix治疗蛇螨,这本来是给人用的,但它也可以用来帮助除去蛇螨感染,因为它能杀死蛇螨和它们的卵。Nix可能比你在宠物店能找到的其他专门的爬行动物螨虫药更有效。然而,用于蛇的使用方法与用于人类的方法非常不同,如果不小心,很容易导致蛇死亡,所以不要按照包装盒上的说明使用。此外,如果你的蛇明显生病,体型小,或者非常年轻,开始治疗之前请先跟兽医讨论治疗方案。
材料
干净的喷雾瓶 56毫升的Nix 1加仑的蒸馏水 适合浸泡的大小合适的盆 操作步骤
将Nix药膏倒入蒸馏水的壶中稀释。摇晃几分钟直到混合均匀。混合后,将溶液倒入喷雾瓶。
如果只在你收藏的一个爬行动物/一个饲养箱中发现了螨虫,那么假设在那个房间里养的所有爬行动物都被螨虫感染了。螨虫也能通过附着在你的手或衣服上转移到其他房间的爬行动物身上。因此,这种治疗必须用于所有的蛇和它们的饲养箱,以确保你不必重复治疗。
将每个爬行动物从饲养箱中取出,放入浸泡盆中。用Nix溶液大量喷洒动物。不要避免喷洒它们的头部、眼睛、耳朵、热坑或其他任何地方——化学药物必须涂抹在所有地方。
在动物浸泡的同时,将饲养箱中的所有底材移除并丢弃。不要在室内进行这个操作——装有感染底材的袋子必须立即放在室外,以防止再次感染。
喷洒整个饲养箱的内外,包括所有的饲养箱家具。特别是要关注角落和缝隙,因为这些地方是螨虫喜欢藏身和产卵的地方。还要在饲养箱周围的地板和/或支架上喷洒2英尺的范围。不要擦拭这些表面——残留的药物有助于防止螨虫的再次出现。
将底材替换为纸或纸巾。在你确信螨虫已经彻底消失之前,使用纸质底材是非常必要的,因为它能帮助你发现可能在初次治疗后存活下来的螨虫。等待3周是安全的时间期限,之后你可以再次使用你平时的底材。
把水碗拿走,水碗和新鲜水应该在24小时后才能放回饲养箱。
将爬行动物放回饲养箱,再次喷洒它、饲养箱、家具和纸。这是为了确保Nix溶液在有机会杀死可能隐藏在爬行动物鳞片下的所有螨虫之前,不会因为在水碗中浸泡而从蛇身上洗掉。
如果/当你的爬行动物在治疗期间排便,移除纸张并清洁区域,但是要在替换的纸和被污染的表面上重新应用Nix溶液。
在7天后重复这个步骤,然后在再过7天后再重复一次,总共进行3次治疗。这样可以确保你杀死了每一个螨虫。
【预防】 参见上面的措施,还是传染病三原则。
网上的图:
https://www.reddit.com/r/snakes/comments/177cqb3/help_snake_is_horribly_infested_with_mites_we/
可能要取样后送药敏测试,根据药敏结果给药
相比其他一些常见的挑食宠物蛇(比如猪鼻蛇和球蟒等),玉米蛇的拒食相对少见。I 当然反过来说如果玉米蛇(在正常时间)拒食,可能反映出更大问题。
Reptifiles
III
如果蛇到了喂食时间但拒绝进食,可以等待半个周期再次尝试(如两周喂食一次,可以等待一周再试一次)。
如果两次都拒食,应该开始寻找原因,可能的原因有:
- 特殊时期(如即将蜕皮,或者刚经历了栖地搬迁/装修,此时拒食可以理解)
- 压力(包括上手过度、不合理共栖等)
- 确认温湿度正确
- 栖地(注意栖地有恰当躲避和遮挡、丰容恰当)
- 食物有问题,比如食物腐败或不新鲜
- 生病
解决:
- 如果是特殊时期,等一等。比如蜕皮导致的拒食再过一周应该能看到蒙眼(in blue)。
- 如果有拿到另外盒子里喂食的习惯,尝试在日常生活的栖地里喂食。
- 如果上手太多(正常时期2~3天一次,每次不超过15分钟),减少上手频率。
- 如果喂的是冻食,尝试活食
- Braining the mouse:方法是找一个一次性针(比如注射器)从面部两眼之前插入,轻轻向后脑方向前进一定距离,达到脑部(注意不是戳穿,也不是把脑搅成浆),然后退出针头。轻轻挤压老鼠头部,使少量无色透明的脑脊液流出(不是挤出脑子),然后即可尝试喂食。喂食前注意洗手,消除手上的鼠味,防止被咬。(Sinclair, 2023
 )III
)III
- 尝试不同种类的食物,比如原来喂小鼠,可以尝试喂带毛鹌鹑苗或日龄鸡崽
- 特殊食品,比如Reptilink的小香肠,或者在老鼠上点上Reptilink的Anole Juice。
- 如果之前没有UVB,提供适当的UVB照射可能能促进进食。(Laszlo, 1969
 )IV
)IV - 注意有无其他疾病,如果有疾病特征,或者做了上述措施都没有用,去看兽医。
【】【强制喂食】【】
注意强制喂食时不能挡住glottis,不然会憋死:https://youtu.be/yh3V3mN33Qs
SnakeDiscovery 20231028的Tinly show视频提到一种解决蛇拒食的方法是让蛇缺水一段(适当长的)时间,使其比较渴。此时用乳鼠沾水,放到蛇的嘴前,蛇会开始喝乳鼠身上的水。这一举动让蛇既能通过“喝汤”尝到乳鼠的味道,也能在乳鼠和正面响应(解渴)之间建立一定关系,有时蛇喝一口水后就会叼老鼠,解决拒食。
(Snake Discovery, 2023 ![]() )I
)I
Anorexia
Failure to feed is a non-specific sign of ill health in corn snakes, but can also been seen as a physiological response in some circumstances. Protracted anorexia (over two months), anorexia associated with loss of body condition or weight, or anorexia in an animal that is clinically unwell merits veterinary assessment. Table 16.2 details some common causes of anorexia.
In all cases a review of husbandry and a thorough clinical examination should be carried out to narrow the differential diagnoses and guide treatment. Where a pathological cause is suspected, but is unclear, further diagnostics including haematology, biochemistry, radiography, and ultrasonography are often valuable. Nutritional support may be applied after rehydration, where significant loss of body condition has occurred but correction of the primary process to enable self-feeding to return is preferred where body condition is not critical.
Table 16.2 Common causes of anorexia in corn snakes.
- Environmental
- Low or fluctuating temperatures:Review temperatures and improve heat provision. If animals are undergoing an intentional cooling period prior to raising temperatures in order to induce breeding then a reduction in appetite is expected.
- Stressors: Assess for potential stressors (e.g. co-habiting with larger corn snake, excessive handling, constant light exposure) and resolve
- Inappropriate prey offered: Prey of inappropriate size, of too low temperature or of unfamiliar appearance or scent may not stimulate a normal feeding response. Offer food of correct size, and of a familiar type that has been warmed to approximately 37 °C. If unsuccessful and the snake is clinically well, try novel, strongly scented items such as hamsters, or freshly killed rodents.
- Overfeeding: Review feeding schedule and quantity fed, as well as body condition of snake. Reduce food if overweight or fed excessively
- Newly acquired snakes: Newly acquired animals, or those recently moved into a new enclosure may temporarily become anorexic. Avoid handling or excess interference for first few weeks of acclimatisation and continue to offer food. Captive bred corn snakes rarely undergo significant anorexia following changes in circumstance.
- Physiological
- Breeding season: Both male and female snakes may become anorexic when reproductively active. Males may become more active. Soft swelling of the mid-coelom may be seen in pre-ovulatory females. Continue to offer food and monitor condition and body weight as these should remain stable.
- Gravid female prior to oviposition: Female snakes will reduce food intake when gravid and may become completely anorexic (Gregory et al. 1999). Offer food once oviposition has been completed.
- Seasonal response: Even if temperatures remain stable other changes, such as external photoperiod, may be perceived by snakes and induce a reduction in feeding in winter. This is uncommon and can be countered by raising usual environmental temperatures by 2 °C.
- Ecdysis: It is common for snakes to refuse food when shedding. Animals will have a blue-grey colour to the skin, which is often most prominent over the eyes. Feeding should resume once shedding has been completed.
- Medical
- Orofacial disease: Stomatitis, traumatic injury, respiratory disease or reduced vision may disrupt normal feeding cues and prehension of food. Physical examination will aid detection and feeding should resume once the primary concern is resolved.
- Debiliation or disease: Anorexia is a common, non-specific symptom in an unwell snake and can be difficult to differentiate from environmental and physiological causes in the early stages. Weight loss or presence of clinical signs of illness support suspicion of a health concern. Clinical examination may identify the cause, but in many cases haematology, biochemistry, imaging and specific pathogen screens may be required to identify the cause.
Reptifile 提前一天准备冷冻的啮齿动物,将其放在冰箱中慢慢解冻。这样可以防止细菌生长,否则会让你的蛇生病。然后,在喂食前,将喂食器放在一个BPA-free的塑料袋中,浸入温热的、几乎热的水中15-30分钟。喂食器的温度应达到约100°F,因为这接近活体啮齿动物的体温。这将模拟体温,鼓励蛇食用。
如果使用冷冻-解冻的猎物,你可以通过摇动喂食器来模拟挣扎,增强你的蛇的“狩猎”体验,并鼓励其取食反应。在蛇攻击并开始收缩后,这可能看起来有些恶心,但这对蛇的精神和身体健康都有利。
总的来说,你必须尊重你的蛇的取食偏好。如果它拒绝吃冷冻/解冻的猎物,就给它活体,并仔细监视它们的互动。活体啮齿动物在你的玉米蛇的围栏中不应超过1小时。保持你的宠物健康和饱食应该始终是你的首要任务。
(Whittaker, 2019 ![]()
![]()
![]() )IV
An alternative could be to create movement of dead prey, for example using a mechanical ball or floating a food bowl in water.
)IV
An alternative could be to create movement of dead prey, for example using a mechanical ball or floating a food bowl in water.
拉稀可能有哪些病?
传染病传播途径
寄生虫的传播:粪口传播,包括人手、用品、昆虫(如蟑螂、小黑飞)、携带寄生虫的食物等。
【】【】【】 原因很多,有的很危险
III
【】【Reddit 有 Regurgitation Procedure】【】
吐食对蛇非常危险,一定要重视预防和治疗。尤其是如果连续吐食两次可能有生命危险。
吐食和粪便的区分:
- 吐食通常还有老鼠的粗略形状,有时有骨骼,取决于吐食前消化了几天。
- 粪便是有点臭,吐食是令人终生难忘的臭(不过某些垫材会缓解臭味)。
- 粪便里有略黄的尿酸,吐食里不会有。
【病因】
- 急性压力:吃饭之后受惊或感到压力,比如吃完饭不久就上手。蛇应激状态下会把食物吐出来以方便逃跑。
- 慢性压力:狭小环境个体中的敏感个体、或不正确共栖关系中弱势个体的慢性压力会影响免疫,导致长期的慢性肠胃疾病,导致吃饱就吐。(冬青-cyan, 2023
 )III
)III - 外界温度不适合消化。温度过冷时甚至可能导致食物在肚子里腐败。消化期间温度过高(如空间狭小且一只加热,缺乏冷区,缺乏昼夜温差)也会吐食。(冬青-cyan, 2023
 )III
)III - 吃太多、太大、太难消化,超过一定时间没有消化时蛇会吐出食物防止食物在体内腐败。(冬青-cyan, 2023
 )III
)III - 有消化系统疾病,如细菌或寄生虫导致的肠炎。有时其他系统的疾病也可能引起压力,间接导致吐食。
- 食物本身有问题
【治疗】
- 一定要重视
- 蛇的吃事物的时候会以特定的方式把骨头顺过来咽下去,吐食时会使得骨头像倒刺一样在消化道内反着走,很可能导致消化道划伤状态。
- 吐食导致肠道菌群环境紊乱。
- 停止上手,必要检查除外。
- 及时检查,及时就医。
- 两周后没有问题可以喂一个最小号老鼠(2~3克);另一说法是喂平时老鼠大小的1/2到1/4。保持不上手,观察情况。
- 如果上述食物被正常消化、排便,可以逐步增大食物的分量,在一两个月的时间内恢复到正常食量。
- 注意保留粪便和呕吐物,就医时化验可能要用。
- 如果二次吐食,必须就医。注意带着粪便/呕吐物。
III Reptifile
【症状】 皮肤轻轻捏起后不会立即恢复原状(而是保持捏起的形状) 眼帽凹陷
【病因】 没水喝(常出现于逃逸找回的蛇) 湿度过低 不愿喝水
【治疗】 【】【Snake Discovery有个视频】【】
治疗脱水的玉米蛇最有效的方法之一是给它进行温暖的电解质浴。
您将需要一个小型热垫,恒温器,几瓶运动饮料或电解质补充剂(如Pedialyte),以及带盖的塑料桶。将热垫放在桶下,设定温度为80-82°F(27-28°C),然后用电解质溶液(运动饮料75%,水25%)填充至2英寸深。让水温上升约15分钟,然后将蛇放入并盖上盖子。让蛇浸泡15-20分钟。
用湿布冲洗掉蛇身上的电解质残留物,然后再将其放回饲养环境中。
自己的视频
https://www.bilibili.com/video/BV1MJ4m1b7gh/?spm_id_from=333.999.0.0 https://www.bilibili.com/video/BV1gN4y1i7Cq/?spm_id_from=333.999.0.0
Frances Baines Admin Calcification of organs does not necessarily indicate a vitamin D3 overdose. Other things can tip the balance between serum calcium and phosphorus which will cause calcium deposition in tissues...Secondary renal hyperparathyroidism comes to mind... I'll do some research and come back tomorrow with some facts.
Frances Baines Admin Otto Mercer First, I want to point out that I think it is extremely unlikely - in fact, I'd say virtually impossible - to overdose a gecko with the vitamin D3 found in a standard commercial gecko diet. Here are my reasons. Two studies show that reptiles, just like humans, are very difficult to overdose with vitamin D3. Doses of 10,000 IU/kg bodyweight were administered to green iguanas (Allen et al 1999) with no adverse effects, all either utilised or excreted within 35 days. The same dose was given weekly to juvenile black-throated monitors (Ferguson et al. 2009) for 3 months, again with no ill effects, even though serum 25(OH)D3 rose to over 600% of normal pre-trial levels. If the 10,000 IU/kg weekly dose was divided into 7 doses that would be about 1,400 IU/day for a 1000g animal, i.e. for a 250g Leachianus, that would be 350IU/day. Repashy Crested Gecko Diet contains only 1600 IU/kg of dry powder. A heaped teaspoon of Repashy dry powder weighs 5g, it therefore contains 8 IU. Now tell me how a meal made from one heaped teaspoon of dry powder mixed with water, fed every day to the gecko, containing only 8 IU of vitamin D3, can cause severe intoxication, if other lizards of a similar weight can tolerate 350 IU per day...
Frances Baines Admin The causes of metastatic calcification are complicated. I've now puzzled my way through two veterinary textbooks and half a dozen online papers and it will take me a while to put together something coherent... but while I do, I think I should point out that we don't know anything like enough about the animal's history to know what had happened to it before it died... What is "soft UV"? Was it fed one commercial diet or many? Were insects fed as well? Were the temperatures appropriate? What about humidity? Could it have had MBD in early life? Could it have suffered from chronic dehydration? Was it male or female? Gravid female? Were vitamin supplements used? Was excessive calcium powder offered? Etc, etc.
Frances Baines Admin
Metastatic Calcification. Otto Mercer OK, this has been a very interesting study. I'll divide this little review into several posts. Metastatic calcification is the name given to deposits of calcium hydroxyapatite (the material with which bones are calcified) that develop in otherwise normal tissue, typically in kidney, lungs and heart, especially the atrial endocardium. The cause is an imbalance in calcium and phosphorus concentrations in the blood, and either excessive calcium or excessive phosphate, or both, can initiate the process. Metastatic calcification in reptiles is most often the result of chronic renal failure (kidney disease), but hypervitaminosis D is also an important cause, usually resulting from an overdose of a vitamin D supplement. Paradoxically, vitamin D deficiency can also cause metastatic calcification... Chronic renal failure is very common in pet reptiles, and a major cause of death. Predisposing factors are dietary protein excess, chronic dehydration due to persistently inadequate humidity or inappropriate water provision, overheating, infection, and nutitional secondary hyperparathyroidism. A diseased kidney fails to filter phosphate efficiently, resulting in an imbalance in the calcium: phosphorus ratio, with increased serum phosphorus. This complexes with calcium producing a fall in ionized calcium, which stimulates the parathyroid gland to produce parathyroid hormone, PTH, to attempt to restore ionized calcium to baseline levels. It acts in two ways; stimulating the activation of vitamin D3 in the kidney, and acting directly on bone. However, a diseased kidney is unable to convert sufficient 25(OH)D3 to its active form, calcitriol (1,25(OH)2D3), which is needed for calcium to be absorbed from the gut, so calcium in the diet is unavailable. To restore calcium levels, PTH secretion increases and stimulates resorption of calcium and phosphate from bone. There is no negative feedback from calcitriol, so parathyroid hormone (PTH) release continues to increase unchecked. Eventually the parathyroid gland hypertrophies - this is 'renal secondary hyperparathyroidism'. The high levels of phosphate in the bloodstream form complexes with calcium, and the mineral deposits form in the soft tissues, especially in the kidneys, around the blood vessels and heart. Calcification in the kidneys continues their destruction.. The diagram below illustrates the process.
Vitamin D deficiency can lead to metastatic calcification in a similar way, in the terminal stages of nutitional secondary hyperparathyroidism. This is because in severe vitamin D deficiency there is insufficient vitamin D for synthesis of calcitriol. Calcium cannot be absorbed from the gut, serum calcium falls, PTH is stimulated to mobilise bone reserves, its production is unchecked, and the end result is as described above.
[Metastatic Calcification 的成因 Frances Baines 95761620_10158365134520127_3876228505993740288_n.jpg]
The occurrence of metastatic calcification following vitamin D3 toxicity is easier to explain. In high enough concentration, native vitamin D can have similar actions upon bone and gut as its activated form, calcitriol, and PTH. Toxic levels will thus initiate resorption of calcium and phosphate from bone, causing hypercalcaemia and hyperphosphataemia, and will also increase calcium absorption from the gut. Hypercalcaemia is thus worsened if the diet is high in calcium. High serum calcium causes renal damage, resulting in further elevation of serum phosphate. The calcium:phosphorus imbalance leads to metastatic calcification. The diagram below illustrates the process.
Metastatic Calcification 的成因2 Frances Baines 96283381_10158365136375127_779853824868220928_n.jpg
There are other factors which may play a part. The situation is exacerbated in females undergoing folliculogenesis, when reproductive hormones stimulate hypercalcaemia and hyperphosphataemia to supply developing eggs with calcium and phosphorus. Vitamin K deficiency is a further risk factor because a serum protein, Matrix Gla Protein, which inhibits metastatic calcification requires vitamin K for activation. High dietary magnesium potentiates vitamin D activities, by increasing its absorption from the gut and increasing its activation in liver and kidney, since the enzymes which convert vitamin D3 to 25(OH)D3 and calcitriol are magnesium-dependent. However, serum magnesium levels are modulated by vitamin D and PTH levels. The effects are complex. Magnesium deficiency can lead to hypercalcaemia and renal calcification, and high vitamin D levels lower serum magnesium; but PTH depletes bones of magnesium as well as calcium and phosphate, and decreases magnesium excretion, thereby increasing serum Mg levels. The role of vitamin A in calcium metabolism is also complex. Vitamin A antagonises vitamin D by competing for nuclear receptors within cells. A high level of vitamin A intake increases the need for dietary vitamin D, and reduces the toxicity associated with hypervitaminosis D. However, high levels of vitamin A have a direct effect on bone and also potentiate the osteolytic action of parathyroid hormone, increasing bone resorption and promoting hypercalcaemia. Finally, we cannot rule out metastatic calcification following rare causes of hypocalcaemia or vitamin D3 hypersensitivity which can occur due to certain genetic abnormalities, cancers or auto-immune diseases. Examples in humans would be Williams' Syndrome, parathyroid adenoma and sarcoidosis. References Agrawal, L., Habib, Z. and Emanuele, N.V., 2014. Neurologic disorders of mineral metabolism and parathyroid disease. In Handbook of clinical neurology (Vol. 120, pp. 737-748). Elsevier. Antinoff, N., 2000. Renal disease in the green iguana (Iguana iguana). Proceeding of the Association Reptilian and Amphibian Veterinarians, Reno, pp.61-63. Divers, S.J. and Stahl, S.J., 2019. Mader's reptile and amphibian medicine and surgery. Mader's reptile and amphibian medicine and surgery, 3rd Edition., (Ed. 3). Doneley, B., Monks, D., Johnson, R., Carmel, B. and Wiley, J. eds., 2018. Reptile medicine and surgery in clinical practice. Wiley Blackwell. Johansson, S. and Melhus, H., 2001. Vitamin A antagonizes calcium response to vitamin D in man. Journal of Bone and Mineral Research, 16(10), pp.1899-1905. Knotek, Z., Dorrestein, G.M., Knotková, Z. and Zwart, P., 2009, November. Chronic renal failure disease in adult green iguanas (Iguana iguana). In Proc. Autumn Meeting BVZS (pp. 7-8). O'neill, W.C., 2007. The fallacy of the calcium-phosphorus product. Kidney international, 72(7), pp.792-796. Raphael BL, James SB, Cook RA. Evaluation of vitamin D concentrations in Uromastyx spp. with and without radiographic evidence of dystrophic mineralization. Proceedings American Association of Zoo Veterinarians (AAZV). Puerto Vallarta, MX, AAZV. 1999:20–2. Walter, JB & Israel, MS (1974). Metastatic Calcification. In: General Pathology 4th Edition, p. 569-575. Churchill Livingstone, Edinburgh and London.
My final comment on this interesting topic is that unless some factor we are unaware of came into play, the evidence presented so far suggests that the gecko is unlikely to have suffered from hypervitaminosis D. The commercial gecko diet contains only low doses of vitamin D3, and fluorescent UVB lamps should not, in theory, be able to promote excessive vitamin D3 synthesis. (And this gecko was provided with a "low level" of UVB from a tried and trusted brand and had free access to shade.) Even strong sunlight (or a fluorescent lamp with a similar UVB spectrum) does not cause toxic amounts of vitamin D to accumulate, owing to the natural "buffering" system in the skin, whereby the precursor 7-DHC is reversibly transformed into several photoproducts, pre-vitamin D3 being only one of them. Relatively inert lumisterol and tachysterol are produced in equilibrium with pre-D3; plus excess vitamin D3, subsequently formed from pre-D3 in the skin by thermal isomeration, can be destroyed by further UV exposure. However, it is theoretically possible, although as yet unproven, that a lamp with an unusual UVB spectrum, very different from that of sunlight, could promote abnormally high conversion of 7-DHC to pre-D3 if its wavelengths were limited to those favouring pre-D3 formation over lumisterol and tachysterol.
【以上为Francis Baines 在 Reptile Lighting Group的表述】
【】【Montiani-Ferreira 2022, Wild and Exotic Animal Ophthalmology.pdf】【】
III
【症状】
- 出血
- 缺磷
- 红肿(常见于丰容不足、环境不适而顶缸导致的吻部损伤)
【原因】
- 顶缸
- 缸内有尖锐物体(如树枝上有木刺、假植的铁丝露出)
- 从高出跌落而没有缓冲
- 逃逸
- 喂活食时猎物反咬或抓伤
【治疗】 Reptifile:
- 用温热的chlorhexidine溶液清洗伤口,并用棉签在受影响的区域涂抹聚维酮碘。抗生素软膏也有用。不用包扎(你根本固定不住)。
- 把土质垫材撤了,换厨房纸等易消毒易清洁的垫材。保持干燥。
- 愈合过程中有蜕皮的话注意卡皮
- 不准泡澡
被猫咬了
https://www.reddit.com/r/cornsnakes/comments/17ib5pg/cat_attacked_snake_pls_help/
四肢肿胀要检查是否有磕碰/外伤 https://www.bilibili.com/video/BV14y421a7Eu/?spm_id_from=333.999.0.0
见喂食章节
Mader's Reptile and Amphibian Medicine and Surgery, 3rd
【定义】
痛风是一种代谢性疾病,直接原因是尿酸的过度生成或排泄不足,导致高尿酸血症、并导致组织中的单钠尿酸(MSU,monosodium urate)晶体沉积。
Neogi T, Jansen TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2015;74:1789–1798.
![[Pasted image 20240415161106.png]]
图:慢性肾脏病爬行动物的体态表现。(A)患有严重肾病和肾性痛风的蟒蛇(蟒蛇)。
【临床重要性】
虽然痛风在人类和灵长类动物中经常见到,但它并不是兽医学中的常见问题。因此,鸟类和爬行类兽医需要参考人类医学文献中关于诊断和治疗的内容。
痛风分三种形式,内脏痛风、关节痛风和围关节痛风。这三种痛风都在爬行动物中常见。
很少有研究记录爬行动物痛风的发病率,但有未发表的数据表明在280例欧洲陆龟的尸检数据中,有64%指示有肾脏疾病,16%指示有痛风的情况(Kolle和Hoffman,2002年,Proc ARAV,第33-35页)。
Vawter RL, Spadaro Antonelli MA. Rational treatment of gout: stopping an attack and preventing recurrence. Postgrad Med. 1992;91(2):115–127.
Wolfe F. Gout and hyperuricemia. Am Fam Physician. 1991;43(6):2141–2150.
Fam AG. Strategies for treating gout and hyperuricemia. J Musculoskel Med. 1988;5(3):83.
【生物化学机制】
在脊椎动物中,尿酸来自于外源和内源性的核酸在肝脏中降解产生游离的嘌呤和嘧啶碱基。如果这些游离碱基没有被身体再利用,它们将进一步降解并最终排泄。嘧啶碱基被分解成最终产物CO2和NH3,这些产物进入鸟氨酸循环(见图66.26)。
![[Pasted image 20240415171234.png]] 图66.26:爬行动物的肝核苷酸、氨基酸和氮元素代谢
在一些脊椎动物中,包括灵长类、斑点狗、鸟类、有鳞目和一些龟类,嘌呤的降解可以产生大量的尿酸。对两种主要的嘌呤来说,腺嘌呤是转化为次黄嘌呤、然后转化为黄嘌呤;而鸟嘌呤直接转化为黄嘌呤。会和至黄嘌呤后,经过黄嘌呤氧化酶形成尿酸。
肉食性爬行动物可以很好的适应高蛋白饮食,在消化中有食性高尿酸血症是正常的;但草食性爬行动物食用大量动物蛋白后可能导致病理性的高尿酸血症。
Campbell JW. Excretary nitrogen metabolism in reptiles and birds. In: Walsh PJ, Wright P, eds. Nitrogen Metabolism and Excretion. London: CRC Press; 1995:147–178.
Hernandez-Divers SJ, Martinez-Jimenez D, Bush S, et al. Effects of allopurinol on plasma uric acid levels in normouricaemic and hyperuricaemic green iguanas (Iguana iguana). Vet Rec. 2008;162(4):112–115.
大多数蛇类和蜥蜴主要通过尿酸代谢方式排泄含氮废物(>90%尿酸排泄),任何减少尿酸肾脏排泄的因素都可能导致痛风。许多龟类,包括陆龟,有较大比例的尿素代谢。水生和海洋爬行动物可能以氨代谢为主。
在爬行动物中,尿酸可以通过肾小球滤过从血液中清除,但主要是通过近端肾小管分泌。尿酸盐的水溶性差,在低浓度下易形成沉淀。虽然尿酸的分泌与肾小球滤过无关,但在脱水情况下,当肾丝球泸过率降低、和肾小管流量减少时,尿酸的分泌会停止,肾小管被尿酸阻塞。在未脱水的情况下,爬行动物的肾单位可以主动分泌超过三倍的尿酸。
Dantzler WH Renal function (with special emphasis on nitrogen excretion). In: Gans C, Dawson WR, eds. Biology of the Reptilia, Physiology A. Vol 5. New York: Academic Press; 1976:447–503.
【致病机理】
在血液中,尿酸主要以单钠尿酸盐(MSU)的形式存在。中性尿酸和尿酸盐在水中的溶解度都相对较低。当这些形式中的任何一种或两种在血液中或其他体液中(例如,滑液)的浓度升高时(如高尿酸血症),MSU会结晶,形成不溶性沉淀物,这些沉淀物会沉积在各种组织中。
滑液中的结晶会导致急性关节炎症,非常疼痛,此即痛风性关节炎。
MSU结晶还可以沉积在关节周围(围关节痛风)和其他皮下及内部组织中(内脏痛风)。MSU结晶会形成小的、肉眼可见的白色结节,称为痛风石(tophi)。
【已知病因】
原发性痛风:尿酸过量产生,通常与高蛋白饮食相关。如给草食性爬行动物喂食过多动物蛋白。又或者为了推动快速生长而过度喂食,野生爬行动物获得食物的频率低于圈养爬行动物,过度喂食是常见的圈养问题,例如痛风是商业鳄鱼生产中常见的问题。
继发性痛风:由于其他慢性疾病或药物导致尿酸生成和代谢平衡被打破。
因尿酸排泄不足导致的继发性痛风:大部分痛风病例涉及脱水(由于环境湿度低、或饮水不足)和/或终末期慢性肾衰竭。
不适温度可能造成肾脏失去工作条件。
圈养压力是肾病的合并因素。
滥用潜在肾毒性抗生素(例如氨基糖苷类和磺胺类)可能导致肾小管坏死,并使患者易患高尿酸血症,尤其是在脱水情况下。
利尿剂是人类药源性痛风的常见原因。如 Furosemide 降低尿酸的肾小管排泄,禁用于脱水、高尿酸血症或疑似痛风的情况。
另有报道在鳄鱼幼体中,维生素A缺乏可能导致肾小管的鳞状化生,从而影响肾脏的尿酸排出,导致尿酸在肾小管中堆积,引起内脏痛风。
Divers SJ. Gout. In: Mayer J, Donnelly TM, eds. Clinical Vet Advisor: Birds and Exotic Pets. St. Louis: Elsevier; 2012:101–102.
Ariel E, Ladds PW, Buenviaje GN. Concurrent gout and suspected hypovitaminosis A in crocodile hatchlings. Aust Vet J. 1997;75:247–249.
【临床表现】
“关节痛风”因单纳尿酸结晶(MSU)在关节滑液和滑膜中的沉积而引起,导致关节炎症。MSU也可沉积在关节周围(围关节痛风)(见图149.1)。
![[Pasted image 20240415160129.png]] FIG 149.1 西非巨蜥(Varanus exanthematicus)的周围关节痛风。注意肘部周围痛风石的积聚和几个手指的肿胀。(Courtesy of Jorge Orós.)
“内脏痛风”指的是MSU在其他软组织中的结晶沉积,如肾脏(见图149.2)、肺、心包、皮下组织、肝脾的浆膜、甚至中枢神经系统中。
![[Pasted image 20240415160140.png]]
FIG 149.2 高冠变色龙(Chamaeleo calyptratus)的肾脏内脏痛风。小图为此动物肾脏中痛风石的组织学表现。放大40倍。(Courtesy of Jorge Orós.)
关节痛风通常先于内脏痛风出现。
MSU结晶的沉积被称为痛风石(tophi),是肉芽肿性炎症组织围绕MSU结晶形成得(见图149.2,插图)。
![[Pasted image 20240415174919.png]] FIG 66.27 尸检:蓝舌石龙子左膝关节的痛风性关节炎. (Courtesy of Stephen J. Divers.)
临床症状通常与受影响的结构有关,可能包括拒食、跛行、关节肿胀和器官功能障碍。
由于痛风导致的循环系统异常以及在神经中形成的痛风石可能导致神经功能障碍。痛风还可能导致中枢神经系统血流减少、进而可能会出现神经疾病症状。
【诊断证据】
体格检查:痛风的推定诊断是基于病史和临床检查作出的。临床表现可能不具特异性,但可能观察到肢体的功能减退或瘫痪、关节肿大、口腔检查可能看到粘膜上的痛风石。
血液检查:实验室采样可能会也可能不会显示出血浆高尿酸血症,这取决于采样时的健康状态。血浆尿酸水平的升高可能具有提示性,但(患者的血浆尿酸)也可能并不显示异常,尤其是关节痛风的患者不一定会有血液检查的异常表现。
超声:MSU沉积物为高回声病变,无后方声音阴影,这有助于将其与钙化区分开(见图66.21)。
![[Pasted image 20240415174429.png]]
图66.21 黄鼠蛇(Pantherophis obsoleta quadrivittata)因高尿酸血症进行的肾脏超声检查。随后的肾活检确认了异嗜性肾炎(heterophilic nephritis)、管状和肾小球退行性变(tubular and glomerular degeneration)、管状矿化(tubular mineralization)和间质纤维化(interstitial fibrosis)。(A) 肾脏横截面视图,左肾测量为1.38厘米 × 0.61厘米。(B) 右肾的矢状视图(Sagittal view)。(C) 整个右肾的矢状声像图重建,总长为13.77厘米。注意所有视图中肾实质中多个高回声点状区域。(Courtesy of Stephen J. Divers.)
放射:X光片可能显示关节周围的溶骨性损伤。软组织中的痛风石的X光吸收差,可能被忽视。慢性的痛风损伤可能会与钙复合矿化,在影像上可见,常常可以在X光片上看到远端关节及其周围肌腱和组织的增强不透明区域。痛风石可能由于骨质侵蚀导致慢性关节炎,并增加了继发感染的风险。
![[Pasted image 20240415161157.png]] 图:患有慢性肾性痛风的网纹蟒的侧面X光照片,展示了由于肾小管扩张和阻塞而产生的沿着肾脏长轴的特征性星芒图案。(Courtesy of Stephen J. Divers.)
CT可以显示晶体聚集,但无法特定识别MSU结晶。双能CT能够将尿酸盐结晶与含钙沉积物区分开来。
内窥镜:内窥镜评估腹膜内器官提供了一种微创的识别病变的方式(见图66.27B和C)。
![[Pasted image 20240415174626.png]]
图:(B) 绿鬣蜥(Iguana iguana)中附着于体腔壁上的痛风石(箭头所示)。(C) 变色龙(Chamaeleo calyptratus)肾脏表面的小痛风石(箭头指示)。(Courtesy of Stephen J. Divers.)
病理:金标准。在受影响的关节或组织中找到MSU晶体。用偏光显微镜检查关节抽吸液(joint aspirates)对识别MSU晶体有用,在偏光显微镜下,尿酸盐晶体呈针状并具有强烈的负双折射。关节液必须在抽取后相对较快进行检查,因为温度和pH值会影响MSU的溶解度。
使用偏光显微镜时,组织必须固定在无水乙醇中,因为如果用福尔马林法的话,尿酸盐结晶在甲醛水溶液固定过程中容易降解或被冲走。当然,即使使用甲醛处理,组织切片仍然可能留下具有特征性的“空洞”,佐以相关炎症可以提示问题的发生。
![[Pasted image 20240415174836.png]] 图:上述变色龙(上方B/C图)的肾活检病理,显示了痛风石(标记为g)的特征性外观。注意,这个用甲醛固定的样本中的尿酸盐物质已经被大量冲洗掉,但所余的空洞仍然可以被用于诊断(H&E染色,原始放大倍数×400)。(Courtesy of Stephen J. Divers.)
真的痛风是MSU结晶形成的。其他不溶物的结晶也可以导致假性痛风(pseudogout),它包括羟基磷灰石沉积症(HADD)、磷酸二水钙盐(CPPD)沉积,同样可以导致急性的关节炎或围关节炎,应当做鉴别诊断,可根据组织学表现、钙的组织化学染色和偏光下的双折射现象来区分。
内脏痛风可以通过传统或内窥镜技术收集的软组织活检的组织病理学检查来诊断(见图66.27D)。
如果没有明显的病变进行活检,则应考虑使用碘海醇清除率进行肾功能评估。
Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30–38.
Jones YL, Fitzgerald SC. Articular gout and suspected pseudogout in a Basilisk lizard (Basiliscus plumifrons). J Zoo Wildl Med. 2009;40:576–578.
Burns RE, Bicknese EJ, Westropp JL, et al. Tumoral calcinosis form of hydroxyapatite deposition disease in related red-bellied short-necked turtles, Emydura subglobosa. Vet Pathol. 2013;50(3):443–450.
【治疗】
输液:纠正脱水和代谢紊乱。可谨慎的使用抗炎药物(必须根据肾功能的评估结果决定)
炎症控制-外科:移除可分离的的关节痛风或周围关节痛风,可能是最有效的治疗方案之一。但结晶形成时,通常已存在严重的关节损伤,并有伴随的永久性关节病变,可以考虑截肢(并合并长期的别嘌呤醇治疗)。亦有从心包移除内脏痛风的做法,可能有益。在孟加拉眼镜蛇(Naja kaouthia)中,单侧肾的内脏痛风通过单侧肾切除术成功治疗。
炎症控制-内科:使用抗炎药物,如秋水仙碱,在必要时可考虑皮质类固醇;谨慎使用NSAID药物,如meloxicam或tramadol,必须排除肾功能不全带来的影响。
疼痛管理:非常重要,人类的痛风极为痛苦,应该使用适当的止痛药管理疼痛。对肾功能不全患者【这种国内不可能买得到的药物】作为止痛剂的效果和安全性可能好于NSAID(非甾体抗炎药)。
抑尿酸合成药物:通过给服黄嘌呤氧化酶的竞争性抑制剂减少尿酸的合成,包括别嘌呤醇(在绿鬣蜥 Iguana iguana 中进行了临床试验,25 mg/kg PO qd 可降低血浆尿酸水平45%,经病例报告确认临床价值;在Mediterranean tortoises(Testudo spp.)中,推荐使用50 mg/kg PO qd x 30天,之后每72小时一次(Kolle Proc ARAV,2001年,第185-186页)。
人类医药中,有些新药,如奥昔嘌醇(oxypurinol)、非布司他(febuxostat,译者注:非布司他并不是黄嘌呤氧化酶的竞争性抑制剂,它是Molybdopterin cofactor位点的非竞争性抑制剂)有良好效果,是人类肾结石、肾功能不全和高尿酸血症的一线治疗药物,安全且耐受性好,副作用少,但是没有爬行动物中的用药报道。
促尿酸排泄药物:sulphinpyrazone, probenecid, benzbromarone(均为参考人类用药)通过阻断肾小管近端上皮细胞(proximal renal tubule epithelial cells)尿酸阴离子重吸收增加尿酸的代谢。然而,这种重吸收在爬行动物中的水平可能非常低,因此这种治疗方法的效果可能不佳。
Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30–38.
Kasper IR, Juriga MD, Giurini JM, et al. Treatment of tophaceous gout: when medication is not enough. Semin Arthritis Rheum. 2016.
Gliozzi M, Malara N, MUscoli S, et al. The treatment of hyperuricemia. Int J Cardiol. 2016;213:23–27.
【预后】
总体来说,严重的爬行动物痛风患者预后差。重度患者通过支持治疗只能维持不长的时间。
严重的内脏痛风预后为差、极差至无望。但仍然有零星的成功案例。
关节痛风的预后取决于关节病变的程度。局部的关节痛风和围关节通风的预后为中等至较差。
痛风在人类中导致剧烈疼痛,应考虑疼痛带来的舒适度和生活质量问题。
【预防】
合理的营养学构成,尤其是提供适量的、适宜类型的(动物性与植物性)蛋白质。
适当提供水分(包括食物含水和饮用水)以防止脱水。
提供维持肾脏代谢所必须的光热条件。
排除圈养中的压力因素,避免因慢性压力导致的肾脏疾病。
在爬行动物用药中,注意药物的肾毒性和对肾脏代谢的影响。
避免维生素缺乏,包括维生素A、B、C、E。
【民间案例】
4月15日:
你好,冒昧打扰下。您在两爬方面的知识和经验丰富,我家鬃狮蜥现在状态非常不好,想请教您的建议,恳请浏览下它的情况。如果您熟悉有这方面经验的异宠医生就更好了。我觉得还没到放弃的时候所以想多方收集下建议,非常感谢。这是他的情况: 鬃狮蜥,1周岁、260g,从3个月大开始饲养。近一周状态堪忧,拒绝进食,无活动,弓背明显,四肢无力无法支撑身体,长时间闭眼,眼眶深陷,腹围增大,体重下降,后腿反射较差。排尿正常。 1月诊断了痛风和肠道寄生虫(脊柱一段骨密度增加、尿酸高;便检有球虫吸虫蛲虫)。服用别嘌醇和驱虫药。 3月份精神情况改善很大,活力度高,进食较积极。 4月初情况开始急剧下降,医生推测是痛风压迫神经造成的神经炎。血液生化结果显示正常钙、磷和尿酸,但高血糖。处理有皮下输液,服用止痛药和大活络丸,喂水,喂食(杂食动物营养粉)。 一直遵医嘱按时吃药和复查,钙和维生素持续补充,期间尾巴有一段弯折,推测是代谢问题或剧烈疼痛导致。 他的生活环境是1.5×0.8×1.5米的陆缸,灯具使用全光谱太阳灯和陶瓷灯,环境温度在25℃~40℃,uvi测试值0~1.4,uvb测试值0~35。
该患者于4月17日死亡
本节资料来源:
- 这篇文献很好:(Schumacher, 2003

 )IV
)IV - (默沙东手册, 2020
 )IV
)IV - (PetMD, 2023
 )III
)III - (Reptifiles
 )III
)III - (Snake Discovery, 2018
 )III
)III - (那个冯柚子, 2022
 )I
)I
呼吸道感染常被缩写为RI(respiratory infection)。
【预防】
- 正确的饲养环境,包括温湿度、光照、栖地设计、共栖关系、水、食物等等。III
- 新蛇一定要注意充分、正确的隔离,至少隔离90天。注意做新蛇的静脉血检和粪检。III
【症状】
有部分下述症状可能指示有呼吸道感染,但具体还需兽医判断,不要自行给药治疗。
-
掰开嘴看口中是否有唾液(掰嘴方法见控制蛇头的正确方法)。正常的蛇掰开嘴后应该几乎看不到唾液(有食物响应时除外);有呼吸道感染的蛇可见拉丝的粘稠唾液。严重者可直接在嘴角上看到吹泡泡现象 IV。下方视频对比了正常的蛇和疑似感染的蛇
有人建议健康蛇应该每三周掰开嘴看一下以尽早发现无外观症状的呼吸道感染(Snake Discovery,2018)。III -
有浆、脓性鼻涕,严重者喷鼻涕泡 IV
-
张嘴呼吸 IV
-
呼吸有异常声音:正常的蛇呼吸时听不到声音(示威喷气时除外);呼吸道感染的蛇常被描述为有”拉风箱声”或“捏爆泡泡纸”的声音 III。
-
蛇想使上半身尽可能伸直、不愿意蜷缩(当然你得给它能伸直的机会,有人的栖地对角线还没有蛇一半长,那它想伸直你也观察不到)。III
-
观察口腔牙齿状况,有无牙龈红肿,是否有口腔炎。III
-
严重者有呼吸困难。III
-
严重者、长时间患病者可导致败血病。III
-
间接症状:蛇状态异常,无力、活动减少,拒食等。III
【诊断】IV
- 病史询问和体格检查,检查是否有上述情况。
- 除上述体格检查外,没有特别好的检查方法,例如:
- 气管灌洗液培养,需要麻醉。
- CT效果还好,但是同样需要麻醉以使蛇伸直。
- X光平片效果不好。 【】【】【】
【直接病因】IV
- 病原体传播,尤其是接触其他病蛇
- 温湿度不适宜,如长期湿度过高
- 通风差
- 卫生条件差
- 吸入异物
- 受伤(如被重物砸伤、肺部的穿刺伤等)
【间接病因】IV
- 长期压力(包括栖地设计和布置问题、也包括不合理的共栖),导致免疫缺陷状态
- 长期营养不良,导致免疫缺陷状态
- 先天问题
- 特殊身体状态,如产卵过后或冬眠苏醒期
【病原体】IV
细菌为主,但病毒、真菌、寄生虫也是可能的病原体。
细菌主要来自气单胞菌属(Aeromonas)、假单胞菌属(Pseudomonas)、变形杆菌属(Proteus)、克雷伯氏菌屬(Klebsiella)和沙门氏菌属(Salmonella)。
具体感染何种细菌可以通过培养气管洗液得出.
如果施用抗生素治疗后短期好转,然后重新恶化,则应考虑合并病毒感染。
有时可以从呼吸道感染的爬行动物中发现真菌感染,但大多数情况下真菌是细菌感染后的二次感染。
寄生虫感染通常伴随细菌和真菌感染
【治疗】IV
- 立即与其他爬行动物隔离。
- 尽快去医院,除非确实没有条件(此处没有条件指的是像新冠疫情管控之类的不可抗力,不包括:没钱、太远、不熟悉宠物医院在哪儿等等不是理由的理由)。
- 修正错误的饲养条件,如不合理的温度、湿度、光照、环境设计、共栖关系、喂食情况。
- 适当提高温度,使温度处于适宜温度区间的上限。提高环境温度可以促进免疫相应(其原理与恒温动物的发烧一致,曾有观察表明受感染的蛇会主动寻找更高温的环境使自己“发烧”),也可以促进呼吸道粘液的排出。
- (通过取样测试确定为细菌感染后)给予系统性抗生素治疗。可选择将抗生素、乙酰半胱氨酸加入稀释的盐水中进行雾化吸入的方式给药,也可结合经口给药。
- 抗生素在爬行动物体内的药代动力学数据很少,通常是已知某种广谱抗生素对爬行动物细菌病原体有效就给动物使用。
- 若使用口服抗生素,注意肠道菌群的重建问题。
- 病毒感染无有效疗法。
- 抗真菌药物在爬行动物体内的药代动力学数据很少,因此其使用成败皆有。因为真菌感染通常伴生细菌感染,所以抗真菌和抗生素治疗通常一同进行。
- 雾化吸入可以使药物直接递送于病变表面,适用于严重、长期感染的爬行动物。
- 雾化吸入每天3~4次,每次20~30分钟。
- 避免过长的雾化治疗,因为可能导致支气管痉挛和肺分流(Pulmonary shunting)。
- 雾化时使用盐水有助于松解粘稠的呼吸道分泌物、帮助清除坏死组织。
- 对严重、长期患病的个体,注意支持治疗。
- 可伴随使用化痰剂、蛋白水解剂和盐水冲洗。
- 应谨慎使用支气管扩张剂,因为有潜在的心血管副作用。选择性β-2激动剂(如Salbutamol, Terbutaline)比非选择性拟交感神经药(如Epinephrine, Ephedrine)要好。
【治疗时间】
蛇的肺炎治疗恢复时间可能很长。
有的可能要六个月(by 王研博医生)。III
有网络案例称用了一个月(by Bilibili 那个冯柚子)。I
【具体用药】IV
- 笔者不是兽医,仅照抄文献。不要自己给药,仅供参考,做为兽医处方的sanity check。
- 细菌感染,因为通常是革兰氏阴性菌导致的感染,常用推荐的广谱抗生素有:
- Amikacin(阿米卡星,5 mg/mL 盐水溶液雾化给药,每日4~6次)
- Ceftazidime(头孢他啶,20 mg/kg 肌肉注射,1~3天一次,可和Amikacin联合施用)
- Enrofloxacin(恩诺沙星,5~10 mg/kg 肌注或口服,0.5~2天一次)
- Piperacillin(哌拉西林,10 mg/ml 盐水溶液雾化给药,每日4~6次)
- Metronidazole(甲硝唑,治疗厌氧菌感染,20 mg/kg,口服,2天一次)
- Doxycycline(兽医黄源,多西环素,5~10 mg/kg,口服,qd,枝原体属)
- 抗真菌药物:
- Itraconazle(伊曲康唑,10~20 mg/kg,口服,qd)
- Ketoconazole(酮康唑,15~30 mg/kg,口服,qd)
- Amphotericin B(两性霉素B,1 mg/mL 盐水溶液雾化给药,bid)
- 抗寄生虫药物:
- Ivermectin(伊维菌素,0.2~0.4 mg/kg,肌注或皮下注射,两周一次)
- Fenbendazole(芬苯达唑,50~100 mg/kg,两周一次)
- Metronidazole(甲硝唑,20 mg/kg,口服,两天一次)
- 抗寄生虫治疗常需要与抗生素治疗同时使用
- 有鼻炎的爬行动物可以通过用洗鼻局部施用1:10的enrofloxacin的盐水溶液
【】【】【】
【】【】【】 (注意不是精神病)
这是神经病吗? http://b23.tv/h7LAjtM
【】【】【】
宠物医院可以接收尸体
【病理检验】
意外死亡后最好进行病理检验,可以了解原因。冬青-Cyan曾提到”中农大的一个朋友“可以为宠物进行病理死因调查(但没有提供其他信息)。(冬青-Cyan, 2021 ![]() )III
)III
【Bilibili念球球】
有时能从体外看到玉米蛇的心跳,这不是异常。从体外看心跳的特点是(王研博医生口述):
- 位于特定位置,如下图所示
- 呈后半身抬高,头部下沉姿态时较为明显,平放时不明显。头较低时回心血增多,起伏会更明显。
- 频率 60 bpm 左右
给蛇采血时有时就需要尾高头低看心跳(如果看不见就用B超观察)
【】【附件/Mader,爬行动物用药剂量和正常血液指标.pdf】【】
【】【https://www.anapsid.org/signs.html】【】
突然死亡
https://www.reddit.com/r/kingsnakes/comments/17kt8g1/im_devastatedlooking_for_answers/
腹部肿物
未知疾病
https://www.reddit.com/r/cornsnakes/comments/1786d6n/am_i_crazy_or_does_she_look_gravid/
https://www.reddit.com/r/cornsnakes/comments/17bs2xx/update_am_i_crazy_or_does_she_look_gravid/
https://www.reddit.com/r/cornsnakes/comments/16a830o/i_think_my_snake_is_going_to_die_please_help/
https://www.reddit.com/r/cornsnakes/comments/17mjztw/corn_snake_pupils_and_headshape_dont_look_good/
https://www.reddit.com/r/cornsnakes/comments/1790xzn/what_could_that_bulge_be/
https://www.reddit.com/r/cornsnakes/comments/16tqfdv/my_boy_seems_to_have_backpain/
https://www.bilibili.com/video/BV1bG4y1e7au/
https://www.bilibili.com/video/BV1vT41137G8/
棕黑锦蛇把肠子拉出来: https://www.bilibili.com/video/BV1XN41137B6/?spm_id_from=333.999.0.0
呼吸道疾病
https://www.reddit.com/r/cornsnakes/comments/16zc6ve/help_sick_snake/
https://www.reddit.com/r/cornsnakes/comments/17edpr9/is_he_ok/
肠炎
https://www.bilibili.com/video/BV1CS4y1i7od/
死亡
https://www.reddit.com/r/cornsnakes/comments/16wcqsi/my_boy_died_lastnight/
鳞片问题
https://www.reddit.com/r/cornsnakes/comments/16zutf4/is_this_scarring_on_top_of_his_head/
https://www.reddit.com/r/cornsnakes/comments/14y6eoe/is_this_scale_rot_or_something_else/
肿瘤
https://www.reddit.com/r/cornsnakes/comments/16mtc1o/my_sweet_girl_passed_away/
气味腺肿
https://www.reddit.com/r/cornsnakes/comments/135051a/impacted_scent_gland/