- 玉米蛇宠物饲养询证指南
- 说明
- 本指南建设原则
- 入手前需要什么
- 基本物种信息
- 解剖与生理
- 动物福利总论
- 蛇的行为学
- 爬行动物的认知能力
- 丰容
- 玉米蛇的自然生境
- 玉米蛇饲养的要素
- 空间需求
- 栖地布置总则
- 光照与加热
- 光照的测试
- 湿度
- 垫材
- 攀爬、遮挡与躲避
- 水
- 食物
- 共栖关系
- 蜕皮
- 特殊状态:冬眠和繁殖
- 互动与行为训练
- 运输
- 摄像头
- 伏地魔栖地
- 应急预案
- 蛇的医疗
- 信息来源
- 其他问题
- 词汇表
- 其他物种
biofilm问题
不要用“往水里扔硬币/金属”的方法防止biofilm https://www.youtube.com/watch?v=ihlZxgomZyc
水除氯剂Reptisafe
给蛇喝的水应当除氯,我直接用矿泉水,如果为了节约成本,也可以用自来水加上恰当剂量的Reptisafe
水盆应该够大,栖地里至少应该有一个水盆可以让蛇泡进去,满足泡澡需求。(泡澡是特殊行为,有时表示栖地内有问题,但有时也是正常的,见这一章节)
不要用透明水盆(如玻璃碗、透明塑料碗)。蛇处理不了透明的概念,看到透明的东西就觉得前面可以走,所在盆里喝完了水就从水下直接往前顶,顶到“结界”又出不去,就转着圈在水下顶,得等到偶尔抬头才能从碗延出去,对蛇造成压力。比如下方这个Reddit视频就生动的展示了这个现象):
这个视频体现出蛇与透明水碗互动时的异常状态:https://www.bilibili.com/video/BV1Xw4m1v74J
一定要用透明水盆时(比如一次性塑料盆)可以选择外面套一个不透明壳子。
供以泡澡的水盆边沿最好不是直上直下的,边上最好有个斜坡,方便蛇找到“出路”:
水盆要够重、够宽,蛇在挖掘时有时会挖到水盆下面并往上拱,小而轻的水盆可能洒一地。
水每日一换,水盆定期消毒(详见清洁和消毒一章)
栖地喷淋/喷雾时经常可以选用纯净水(或反渗净化水),这样可以避免留下盐渍;但是蛇的饮用水不要用纯净水,而是应该含有合理的矿物质。但是缺乏矿物质会在蛇体内食入后产生渗透压失衡。长此以往,即使蛇经常喝水,这也可能实际上导致脱水。
【】【Reptisafe水处理剂】【】
较深的水盆应该有个斜坡出口【】【伏地魔的水盆】【】,因为蛇更喜欢尝试从水平方向脱离水盆。如果水盆又深、又只有垂直的壁,蛇可能要拿鼻子转着圈顶半天才知道抬头。如果有个斜坡那想走就直接出去了。
脱水的处理见蛇病-脱水章节
蛇有几种饮水来源,一是水盆,二是喷淋时粘在物体表面的水,三是进食时会同时摄入水
加湿喷淋用的水应达到(蛇的)饮用标准,不要使用未除氯的自来水加湿。因为蛇有时会在“下雨”时饮用叶片、墙面上掉落的水滴(这是一种野外生存策略,因为野外的固定水源都很脏,但下雨粘在物体表面上的水比较干净)。如果使用经Reptisafe等除氯剂处理的自来水(而不是现成的饮用水或蒸馏水)做喷淋,应使水呈较大水滴状态喷淋,避免过分雾化。雾化的除氯水中的可溶物会挥发形成悬浮无机盐粒子污染物,可能导致肺炎、还使栖地玻璃结盐渍。
蛇经常会在进食前后饮水,有时喂食前会水洗蛇进食的餐具(比如我用翻过来的一次性盘子喂食,有人把蛇拿出栖地喂食),此时应注意用饮用水涮洗这些器具,让剩下的水达到饮用标准,甚至可以故意再加一点饮用水,否则蛇可能在进食前后去喝容器上沾的自来水。
不大建议给蛇蛇用透明水盆。蛇进化过程中没见过竖着的透明物体,所以很多蛇理解不了透明的墙。
使用透明水盆时蛇如果想从水盆里出来,它可能觉得前面啥也没有,直接往前走就行,然后就撞墙。他就纳闷这怎么结界了呢?然后就只能用触觉试探,体现为拿鼻子点墙。这个视频最后两秒钟就是开始拿鼻子点墙了,这是很典型的想走了找路呢,估计视频结束后还会继续点墙。
【大蚯蚓饮水-哔哩哔哩】 https://b23.tv/BNiCEeh
如果水碗浅还好一点,他顶了墙继续用力就会自然抬头,然后就知道边沿在哪儿了;水碗深得时候可能折腾半天也找不到,就会造成压力。“紧急拉屎”有的时候也是蛇感到外部压力时的一种应激响应(当然有的时候也不是)。
最好能换个不透明的浅盆给它泡澡,面积和水深要足够蛇平着盘进去泡澡,但水深也不要超过蛇厚度的两三倍,边沿最好有个缓坡(不是直上直下,类似图上这样,只是说明样式,不是广告),让他能自然的用视觉找到出路。
[ BV1eP411a7NB ] 冬青动物志 :[18:44] 蛇拒食的原因有很多,有蛇的原因 也有食物的原因,这期我主要讲一下后者(因为后者少有人讲),要知道 冷冻本身也会破坏营养,冻得越久(营养)流失得越严重,超过半年 就会变成“僵尸肉”,这种食物在解冻后,有些蛇就能感受到变质 从而拒食,所以冻货不要屯太久,圈养食物本来就单一,长期食用维生素流失的食物会增加患病风险,而室温解冻 或者直接泡开水,也可能让食物闻起来更不新鲜,所以最好是先放冷藏环境下彻底解冻,喂之前再用热水或微波炉,迅速加热到30度左右,再立刻擦干投喂,这样对营养的影响是最小的,其实这两点 对人类也适用,这个生活小常识 你记住了吗
应当记录体重和食物重量,需要精确至0.1 克(尤其对于小蛇)的厨房秤,不要使用只精确到1克的厨房秤。
做食物丰容时使用scent trail可以大大增加蛇的运动量(Reptiles and Research),可以用多只小鼠分散于栖地各处,并拖拽老鼠形成scent trail。蛇会不断探索栖地(尤其是它不知道一共有多少老鼠,所以它不知道啥时候是找完了)。因此即使已经吃完了所有老鼠,还是会转很长时间。这不但增加了你看到蛇的概率,还增加了蛇的运动量和活动丰富性。
【】【未整理】【】
K. Arbuckle曾经发表过一篇用一日龄的鸡仔喂蛇的论文【】【】【】,这是通过询证饲养的思路否定“民间饲养法”长久错误观点的例子。作者通过科学的实验设计发现用鸡仔作为主粮喂蛇不但没有造成营养问题,还有助于解决许多球蟒的拒食问题。并且鸡仔其实比等质量的小鼠、大鼠便宜,即使蛇吃了鸡仔之后不再吃老鼠也不是大问题。鼠确实有比鸡仔更高的钙,但只要营养本身能达标,稍少一点并不会造成营养问题,尤其是多种食物混合喂养的时候。混合喂养还增加了食物丰容。
自己写的回复: 不可以长期喂牛肉。蛇会消化鼠、鸟的骨骼,并且需要毛和羽来做膳食纤维,蛇拉出来的屎除了尿酸之外就只有未消化的毛形成的“毡子”。如果长期喂牛肉会缺乏消化骨骼带来的营养,其中最主要的是钙磷比过低。(视频中喂褪毛鹌鹑其实不太好,不能长期这么喂,最好带毛;而且不宜喂一大堆小的,蛇的胃是被设计来要“撑粗了”来消化的,现在视频中一大堆小的撑不粗,同样重量下在蛇体内就排的很长,长此以往容易消化不良或吐食。当然偶尔、适量也问题不大,蛇在野外也会偶尔一下干掉一窝雏鸟。)
玉米蛇完全肉食。
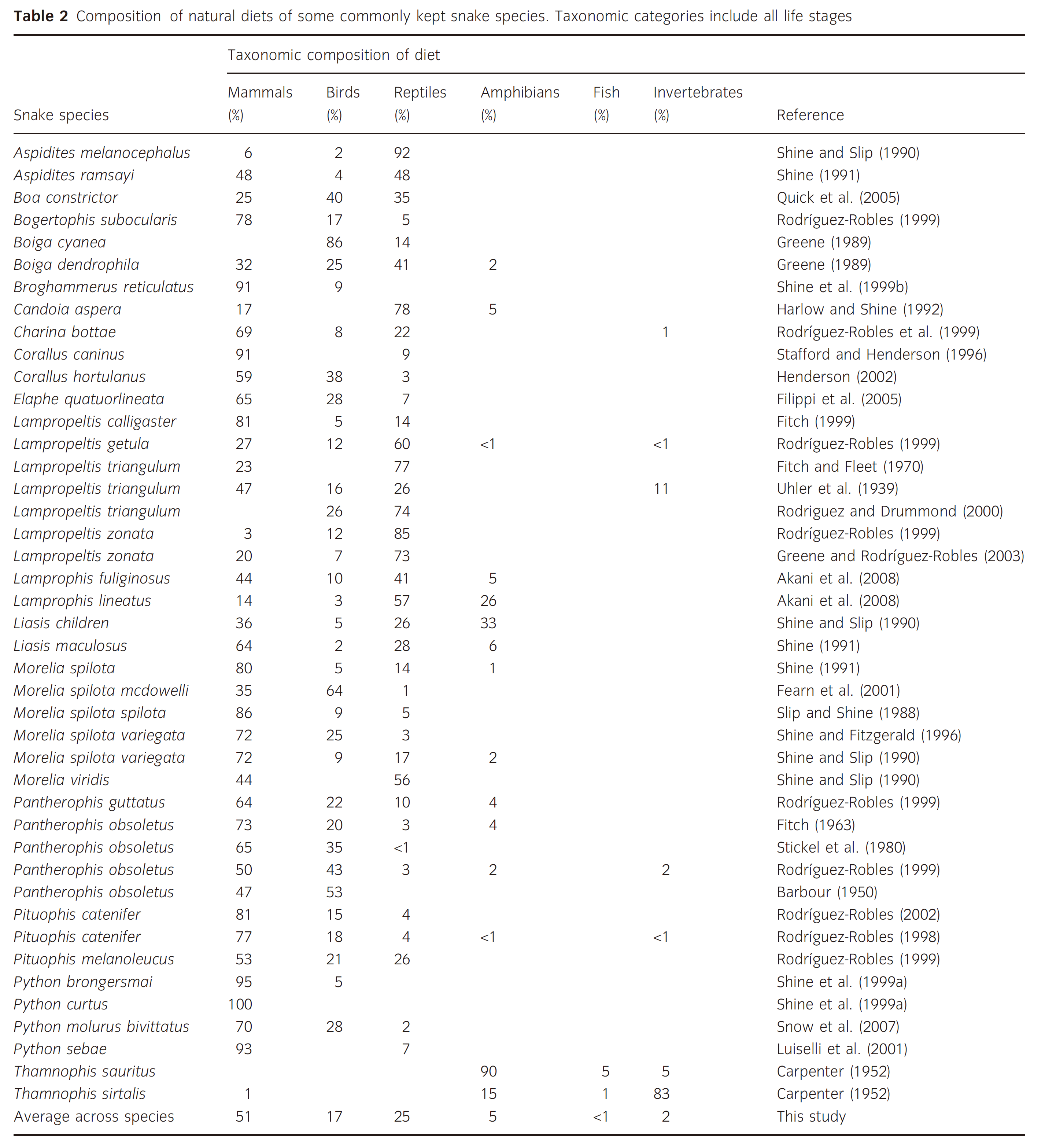
玉米蛇并不是只吃老鼠,野外食物构成有(Arbuckle, 2010 ![]()
![]() )IV ,(Rodríguez-Robles, 1999
)IV ,(Rodríguez-Robles, 1999 ![]() )IV :
)IV :
- 哺乳类:64%
- 鸟类:22%
- 爬行类:10%
- 两栖类:4%
实际上,绝大对数蛇都不是只吃一个类群的食物:
玉米蛇只吃老鼠确实可以活,但说玉米蛇就是应该只吃老鼠、甚至说玉米蛇吃鸡、吃鹌鹑会造成营养问题是错误的。
有文献通过给900英亩范围内的206个鸟巢(共13个鸟类物种)安装摄像机详细记录了97起玉米蛇的鸟类捕食行为(其他蛇的捕食行为有40起,说明在相应区域玉米蛇是幼鸟的主要猎食者)。(Degregorio, 2016 ![]() )IV
)IV
研究发现:
-
玉米蛇夜间捕食,抄巢捕鸟的时间绝大部分在20:24到24:00之间
-
玉米蛇捕食巢中有幼鸟的鸟巢的概率是巢中有蛋的鸟巢的概率的5倍,说明玉米蛇可能白天就看好了晚上要去干哪个鸟巢。
-
可以观察到玉米蛇多次(4次)在其他蛇抄巢之后就去巢里捕食,说明玉米蛇可能可以通过观察其他蛇的行为决定自己的行为。
-
虽然观察中的玉米蛇大小足够吃下成鸟,但没有观察到玉米蛇攻击成鸟的行为,只吃幼鸟。虽然不直接攻击成鸟,但有把睡着的成鸟用头推出巢的行为,也有钻到成鸟下面捕食的行为。
-
相比只有蛋的巢,玉米蛇有4.5~5倍的概率攻击有雏鸟的巢。
-
玉米蛇攻击的鸟巢既有森林边缘的,也有森林深处的。(不要因为herping的时候玉米蛇主要被发现于草地和路边,就觉得玉米蛇就只爬草不上树)
-
玉米蛇每次抄巢的时间在28~45分钟(食物丰容时可以考虑用各种方法增加捕食时间,不要让捕食行为十秒钟就结束了。)
摄像机覆盖的鸟巢有:Cardinalis cardinalis(85巢),Passerina caerulea(25巢),Toxostoma rufum(27巢),Passerina cyanea(19巢)
用于参考的成体体重(不过注意玉米蛇吃的大多是刚孵化的幼鸟): Cardinalis cardinalis:33-65 g Passerina caerulea:26-31.5 g Toxostoma rufum:61-89 g Passerina cyanea:11.2–21.4 g
(Nijboer, 2022 ![]() )IV
)IV
Nutrition in Reptiles By Joeke Nijboer , PhD, Nijboer Consultancy Reviewed/Revised Aug 2020 Modified Oct 2022 Vitamin D and Ultraviolet Light Prey For More Information Topic Resources 3D Models (0) Audios (0) Calculators (0) Images (0) Tables (3) Videos (0) Also see Management of Reptiles.
Appropriate husbandry of reptiles is as important as providing adequate nutrients See table: Composition of Animal Foods that May Be Offered to Reptiles. Photoperiod, temperature, humidity, substrate, stress, and cage “furniture” can affect feeding behavior and, thus, nutrient intake. Temperature and humidity gradients within a reptile enclosure allow the animal to select warm, dry spots or cooler, moist areas. Competition for preferred sites and for food pans in an enclosure with multiple animals should also be assessed. Sufficient numbers of warm spots, UVB exposure spots, and food pans should be available for all animals within an enclosure. Visual barriers may be useful to reduce competition for preferred sites or food dishes.
Prey such as rabbits, rats, or mice should come from commercial breeding centers and be offered dead to prevent injury to the reptile and for welfare reasons of the offered prey. Although it is not common, prey have been known to attack predators and can inflict serious bites. Offering dead prey can also reduce the chance of injury to the predator caused by striking the walls of the enclosure. However, some reptiles may initially need the stimulation of live prey, particularly if they are not adapted to captivity. The possibility of disease or parasite transmission from prey to predator should be considered.
TABLE
Composition of Animal Foods that May Be Offered to Reptiles
Vertebrate prey should be fed nutritionally complete diets appropriate for the species (eg, mouse diet, rabbit diet, rat diet, etc). The nutrient content of the prey depends on what it is fed (eg, mice raised on a diet deficient in vitamin A have decreased liver storage of this essential nutrient). Additionally, if frozen mice or rats are routinely used to feed carnivorous reptiles, freezer storage conditions should be optimal (eg, ≤6 months and in thick, plastic bags to retard deterioration, stored at -20°C). Methods of thawing that minimize water loss are also important. Because many carnivorous reptiles rely on their prey not only as sources of nutrients but also as sources of water, the state of hydration of the prey can be very important. Thawing should be done in a cooler at < 8°C.
Familiarity with a species’ food habits in the wild is essential if appropriate foods and nutrient levels are to be offered. Common practice has been to offer two or more different prey species, because differences in nutrient content exist among vertebrate and invertebrate prey. Reduced dependence on a single food or prey species is also desirable, because some prey items may be periodically difficult to obtain. Dependence on a single prey item is frequently seen in snakes and may be unavoidable.
Many commercial diets for reptiles are marketed. Products for carnivorous, herbivorous, and omnivorous reptiles are now available in frozen, freeze-dried, canned, extruded, pelleted, or sausage forms. Acceptability may be better when the commercial diets are offered to reptiles when they are young. Appropriately formulated, manufactured diets for reptiles are a potentially simpler and more economical alternative to feeding fresh produce or live prey. However, some of these diets may not be formulated rationally, and frequently little information concerning micronutrient concentrations is provided by the manufacturers. When selecting a commercial product, the buyer should obtain accurate information about product formulation and specific nutrient concentrations. Unfortunately, little controlled research has been conducted on nutrient requirements of reptiles, and claims of product superiority may not have a scientific justification. ( See table: Composition of Animal Foods that May Be Offered to Reptiles)
Herbivorous reptile pellets should make up 25%–50% of the diet of herbivorous reptiles. Animals should be fed 1%–4% of their body weight on a dry-matter basis. Vegetables with a low amount of oxalate should be fed to prevent kidney stones. A good quality grass hay or a so-called herbs-hay should be fed. No more than 50% of the diet should consist of fresh greens, fruits, and vegetables. The amount of fruit should be no more than 5%. In Europe, often herbs and dandelions are fed to herbivorous reptiles. Fresh, clean water must be available at all times.
See table: Recommended Nutrient Concentrations for Reptiles for recommended nutrient concentrations for reptiles.
TABLE
Recommended Nutrient Concentrations for Reptiles
Vitamin C synthesis has been reported in many reptile species. It has been suggested that ulcerative stomatitis seen in snakes and lizards may be associated with a vitamin C deficiency, although there is no supportive evidence. In controlled studies with garter snakes (Thamnophis sp) fed supplemental vitamin C, tissue levels and body stores remained stable, although synthesis by the snakes was reduced.
Although most reptiles excrete nitrogen primarily as uric acid, aquatic reptiles typically excrete excess nitrogen as urea or ammonia. The relative proportions of various nitrogenous wastes may depend on the amount and composition of feed, frequency of feeding, and state of hydration. The excessive precipitation of urate crystals in joints, kidneys, or other organs (gout) can be a common condition in some species of captive reptiles. The etiology is not clear, but it is commonly thought that diets high in protein may predispose reptiles to gout. Impaired renal function and dehydration have also been suggested as possible causes.
If poor-quality protein is fed (unbalanced amino acids) or when tissue is catabolized for energy, uric acid excretion increases. Although gout in some reptiles is associated with increased circulating levels, postprandial transient increases in circulating uric acid may be seen in some species and confound the diagnosis. Assuring an adequate state of hydration in a susceptible animal may help prevent uric acid precipitation in joints and organs. Feeding diets low in protein to carnivorous reptiles is unwise, because they are adapted to feeding on high-protein prey.
Vitamin D and Ultraviolet Light for Reptiles Most vertebrates can either absorb vitamin D from the diet or synthesize it in the skin from 7-dehydrocholesterol using energy from ultraviolet (UVB) light of certain wavelengths (290–315 nm) in a temperature-dependent reaction. Thus, vitamin D is required in the diet only when endogenous synthesis is inadequate, as develops when animals are not exposed to UV light of appropriate wavelengths.
Many captive basking species appear susceptible to rickets or osteomalacia (metabolic bone disease). Bone fractures, soft-tissue mineralization, renal complications, and tetany can develop. Reptiles frequently show few premonitory signs, although lethargy, inappetence, and reluctance to move are commonly reported. Serum calcium concentrations may not be diagnostically useful. Although blood levels of vitamin D can be measured, normal values for most species are not known. Supplementation with injectable calcium and vitamin D may provide some short-term relief. However, exposure to UV light, or lack of it, may be an important, yet often overlooked, factor in the differential diagnosis. Complicating the diagnosis may be soft-tissue mineralization, seen radiographically or at necropsy.
In green iguanas, metastatic calcification may not result from vitamin D toxicity. Iguanas with both fractured bones and extremely low or undetectable levels of circulating 25-hydroxycholecalciferol also had calcified soft tissues. The etiology of the metastatic calcification is not understood and is contrary to conventional understanding of the signs of vitamin D deficiency and toxicity in domestic species. Dietary sources of vitamin D may not be sufficient to prevent rickets and osteomalacia. Diets with as much as 3,000 IU vitamin D3/kg did not prevent bone fractures and cortical thinning in green iguanas. Bulbs emitting UVB placed over the lizards at ~12–18 in. for 12 hours/day appeared to reverse the signs in the least severely affected lizards.
Because some lizards seek a warm spot to increase body temperature, placement of a warming bulb, usually incandescent, adjacent to a UVB bulb helps ensure adequate exposure to UVB light. Exposure to unfiltered natural sunlight, depending on latitude, during warmer months and use of UVB bulbs during the rest of the year usually eliminate the risk of bone disease caused by insufficient absorption of calcium (due to a vitamin D deficiency). Some reptiles can accumulate 25-hydroxycholecalciferol when exposed to UVB emission of bulbs, so UVB exposure every day is not necessary, but it is not clear yet how much exposure is needed and how much time can pass before another UVB bath is needed.
Some lizard species may be unable to absorb sufficient dietary vitamin D3, although the reason is poorly understood. New World primates are believed to have exceptionally high dietary requirements for vitamin D, which may be related to lower numbers of vitamin D cellular receptors than are present in Old World primates. Similar metabolic differences may exist in some basking lizard species, although this has not been established. UVB bulbs are sold in pet stores, but label claims may not be reliable.
UVB Lighting Three types of UVB lighting are on the market: fluorescent tubes, compact fluorescent lamps, and mercury lamps. Fluorescent tubes supply a diffuse light with a low amount of visible light. Heat radiation is low, and the UVB gradient is fairly uniform. The light from fluorescent tubes resembles more or less the natural UVB in the shade of a sunny day spread over a relatively large area. Compact fluorescent lamps provide a more intensive UVB gradient focused on a small area. These lamps are characterized by fairly low intensity visible light and little heat. Mercury lamps (vapor spot and narrow spots) produce an intensive UVB gradient on a smaller area, producing heat and an intense light.
Mercury lamps can become very hot. Reptiles must be prevented from getting burned during UVB basking. It is important to recognize that when a UVB lamp is added to a terrarium, the emission of UVB drops with the square of the distance; this explains the low exposure level of UVB at the level of the reptile when the lamp has been hung too high.
The radiation of UVB declines during the lighting time. In general, UVB lamps should be replaced once a year. However, it is best to regularly measure the amount of UVB with meters used in the artificial sunbath industry. A “D3 Yield Index” that compares the vitamin D3–producing ability of the lamp with the sun has been developed, and the results show that there can be huge differences between UVB lamps, which according to the manufacturers should emit high amounts of UVB.
Tests have been done with LED-emitting UVB lights showing that the optimal wavelength for synthesizing provitamin D in the skin is 293 nm. Some products containing UVB LEDs are now on the market. More products with UVB LEDs will certainly be marketed, and more research will be done on the effects of UVB LEDs on reptiles and other animals such as birds, primates, and other mammal species. An important consideration is that UVB LEDs are expensive and the amount of emitted of UVB is high, which can be toxic if an animal is overexposed.
How long and how much exposure to UVB is needed in reptiles is not exactly known. In general, reptiles that need UVB must be exposed to 30 minutes to 2 hours of UVB each day when older types of lamps are used. It is still unknown how much UVB reptiles and other animals should be exposed to when using modern LED UVB lamps. Enlisting the assistance of a specialist is advised, because there is no ideal UVB bulb yet ( see Environmental Lighting).
For example, bearded dragons do not develop metabolic bone diseases if they are exposed only a few times a week to UVB for a limited time. Similar effects of UVB may also apply to other reptiles; more research is needed.
Prey
TABLE
Proximate Analysis of Whole Prey
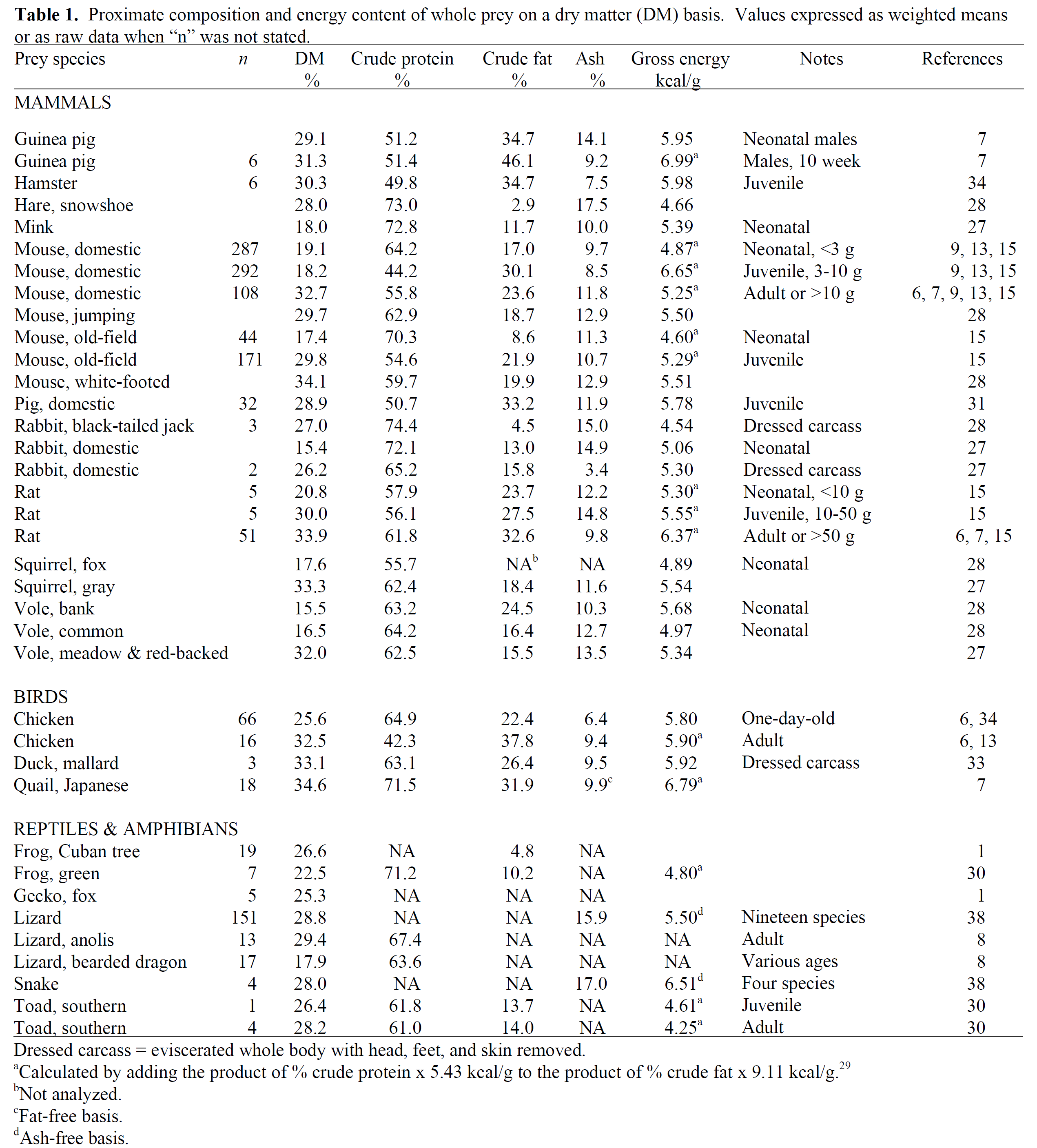
Many reptiles, as well as some birds and mammals, are fed prey. The prey can consist of different species of rodents, birds, insects, and larvae. The analyses on the fed prey comes from research publications and anecdotal literature and is scattered. A lot of analyses are performed on a single prey, which means that most are not statistically validated analyses. Also there can be a variation in analysis techniques. In the associated table (Proximate Analysis of Whole Prey), where no value is added it means that no data are available. The table is a summary of the most important values on prey. More information (eg, on fatty acid, amino acid and vitamin and mineral composition on several prey species can be found on the Feedipedia website.
Composition of Animal Foods that May Be Offered to Reptiles
| Food Item | Dry Matter (%) | Protein (%) | Fat (%) | Energy (Kcal/g) | Calcium (%) | Phosphorus (%) | Ca:P Ratio |
|---|---|---|---|---|---|---|---|
| Chicken muscle | 25.6 | 20.5 | 4.3 | 1.21 | 0.01 | 0.2 | 0.05 |
| Egg whole | 25.2 | 12.3 | 10.9 | 1.47 | 0.05 | 0.22 | 0.02 |
| Mice, 1-2 days old | — | — | — | — | 1.6 | 1.8 | 0.88 |
| Mice adult | — | 19.86 | 8.81 | 2.07 | 0.84 | 0.61 | 1.37 |
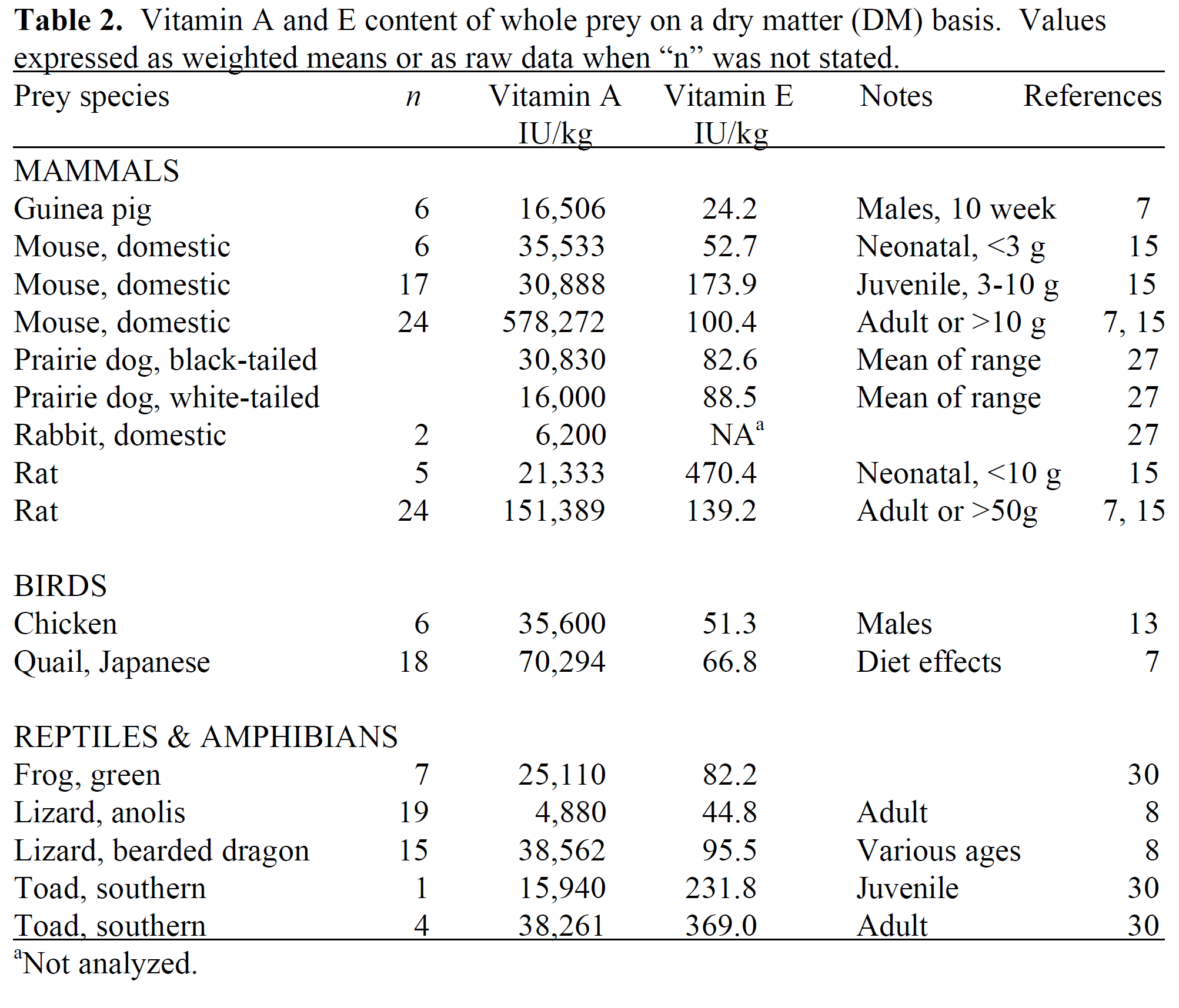
Recommended Nutrient Concentrations for Reptiles
| Nutrientb | Carnivorous Reptiles |
|---|---|
| Crude proteinc | 30%–50% |
| Fat | |
| Crude fiber | |
| Arginine | 1.00% |
| Isoleucine | 0.50% |
| Lysine | 0.80% |
| Methionine | 0.40% |
| Methionine + cysteine | 0.75% |
| Threonine | 0.70% |
| Tryptophan | 0.15% |
| Linoleic acidd | 1.00% |
| Calcium | 0.8%–1.1% |
| Phosphorus | 0.5%–0.9% |
| Potassium | 0.4%–0.6% |
| Sodium | 0.20% |
| Magnesium | 0.04% |
| Manganese | 5 ppm |
| Zinc | 50 ppm |
| Iron | 60–80 ppm |
| Copper | 5–8 ppm |
| Iodine | 0.3–0.6 ppm |
| Selenium | 0.3 ppm |
| Riboflavin | 2–4 ppm |
| Pantothenic acid | 10 ppm |
| Niacin | 10–40 ppm |
| Vitamin B12 | 0.020 ppm |
| Choline | 1,250–2,400 ppm |
| Biotin | 70–100 ppb |
| Folacin | 200–800 ppb |
| Thiaminee | 1–5 ppm |
| Pyridoxine | 1–4 ppm |
| Vitamin Af | 5,000–10,000 IU/kg |
| Cholecalciferol (vitamin D3)g | 500–1,000 IU/kg |
| Vitamin Eh | 200 IU/kg |
a Nutrient concentrations are recommended minimums for carnivorous reptiles and averages for omnivorous reptiles.
b Nutrient levels expressed on a dry-matter basis.
c Taurine requirements have not been determined for reptiles (the requirement for cats is 400–500 mg of taurine/kg dry diet).
d A dietary source of arachidonic acid at 200 mg/kg dry diet may be necessary.
e Thiamine concentrations should be increased to 10–20 mg/kg if frozen, thawed fish constitute >25% of the diet offered.
f A source of preformed vitamin A may be required because it is not known if reptiles can convert carotenes to retinol (vitamin A), although it is likely that herbivorous reptiles can.
g Requirements for vitamin D may be partially or totally satisfied by exposure to sunlight or appropriate sources of artificial ultraviolet light. These suggested concentrations are not sufficient to prevent signs of vitamin D deficiency in green iguanas.
h 300 IU/kg dry matter is advisable if the diet is high in fat, especially unsaturated fat.
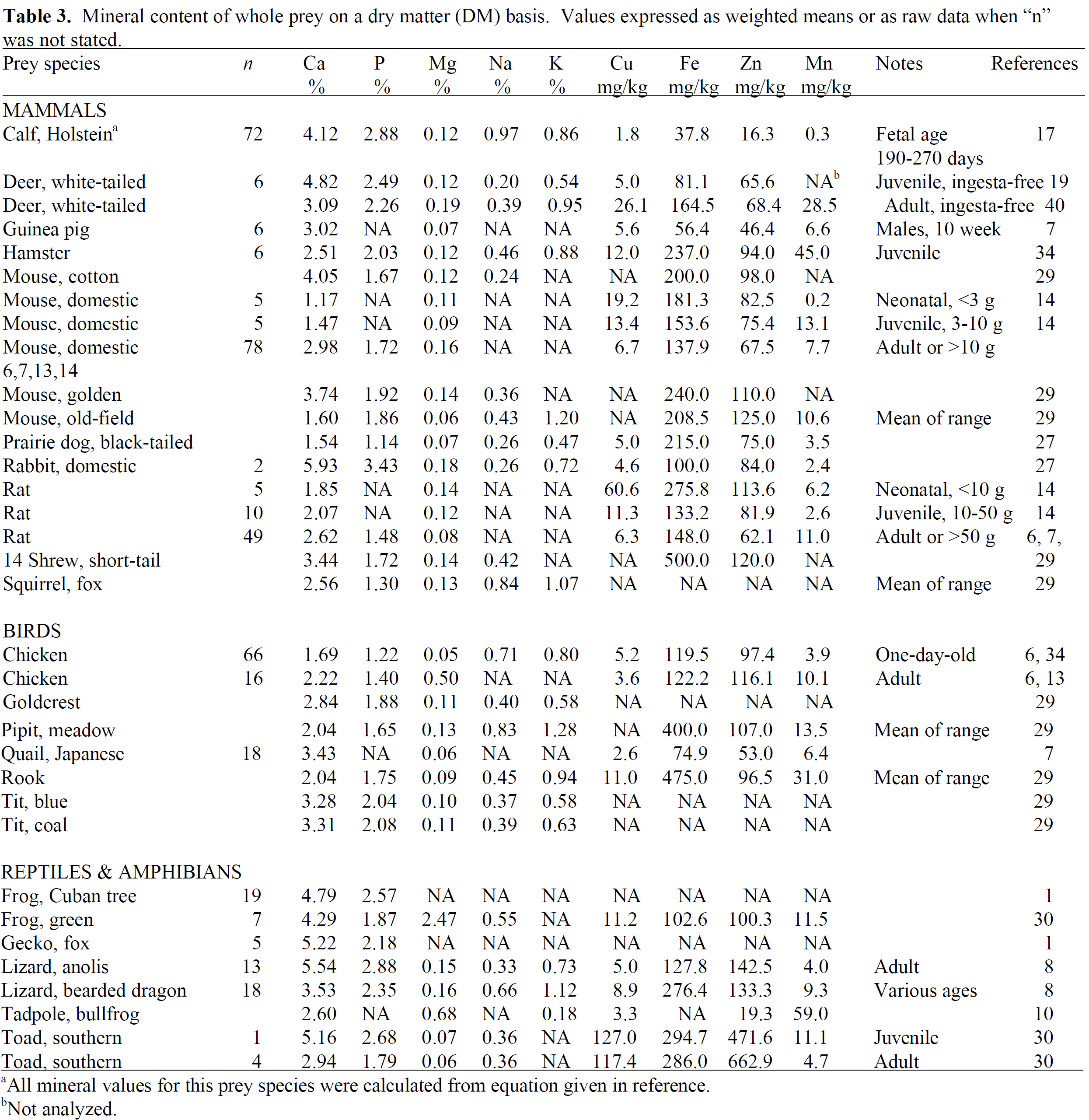
Proximate Analysis of Whole Prey
| Species | Scientific Name | Dry Matter (DM) | Crude Protein (% in DM) | Crude Fat (% in DM) | Ash (% in DM) | Ca (% in DM) | P (% in DM) | Cu (mg/kg DM) | Fe (mg/kg DM) | Zn (mg/kg DM) | Mn (mg/kg DM) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rodents | |||||||||||
| Rat, adult | Rattus norvegicus domestica | 29.8 (20.8–34.4) | 59.7 (56.1–62.8) | 26.5 (22.1–32.6) | 11.7 (9.8–14.8) | 2.8 (2.1–3.5) | 1.7 (1.5–1.9) | 4.5 | 58.9 | 43.3 | |
| Rat, baby | 20.8 | 65 | 20.9 | 1.7 | 1.1 | 27.5 | 171.5 | 106.7 | 5.2 | ||
| Rat, 11 weeks | 35.7 | 63.4 | 34.9 | 7.5 | 2.3 | ||||||
| Mouse, adult | Mus musculus | 281. (18.2–35.6) | 54.9 (44.2–64.2) | 28.4 (17.0–46.5) | 9.6 (7.6–11.8) | 1.9 (1.2–3.0) | 1.5 (1.2–1.7) | 11.8 (6.7–19.2) | 139.4 (34.6–181.3) | 68.3 (47.7–82.5) | 6.3 (0.2–13.1) |
| Mouse, baby | 20.7 | 68.7 | 20.1 | 9.9 | 1.5 | 1.8 | 22.4 | 247.6 | 103.6 | 4.8 | |
| Mouse, 25–35g | 40.4 | 56.1 | 27.1 | 9.7 | 3.5 | 1.8 | 14.7 | 204.7 | 0.4 | 7.8 | |
| Guinea pig, adult | Cavia porcellus | 33.6 | 46 | 39 | 11.1 | 2 | 3.1 | ||||
| Guinea pig, baby | 29.1 | 51.2 | 34.7 | 14.1 | |||||||
| Guinea pig, 10 weeks | 31.3 | 51.4 | 46.1 | 9.2 | 3 | ||||||
| Rabbit, adult | Oryctolagus cuniculus domestica | 31.8 | 59.9 | 22.5 | 14.2 | 2.3 | 2.3 | ||||
| Birds | |||||||||||
| Day-old chick | Gallus domesticus | 25.7 (25.0–27.7) | 64.4 (60.0–72.4) | 24.1 (22.4–28.1) | 7.1 (6.4–7.4) | 1.3 (0.8–1.7) | 0.9 (0.5–1.2) | 2.6 | 52.3 | ||
| Chicken, whole | 33.5 | 43.1 | 35.2 | 12 | 2.7 | 2.1 | 3.6 | 122.2 | 11.6 | ||
| Chicken leg | 40.3 | 49.9 | 36.7 | 6.4 | 1.7 | 2.7 | |||||
| Chicken liver | 26.3 | 81 | 10.5 | 5 | 0 | 1.2 | 18.7 | 358.8 | 125.6 | ||
| Quail | Coturnix japonica | 34 | 63.7 | 28.7 | 10.3 | 3.7 |
(Nijboer, 2022 ![]() )IV
)IV
Snakes feed almost exclusively on vertebrate or invertebrate prey. A few species are specialized egg feeders. Most boids, pythons, vipers, colubrids, crotalids, and elapids are fed mouse pups, mice, chicks, hamsters, rats, guinea pigs, chickens, ducks, or rabbits. Frozen, thawed prey are usually used in zoos; thawing under refrigeration is recommended. After thawing, prey should not be fed cold but at room temperature, or preferably warmer. Some species (eg, king cobra, hognose snake, garter snake) feed primarily on other poikilotherms in the wild. Some of these species can be switched, at least in part, to homeothermic prey, which is often more available and less expensive.
Minced prey is sometimes fed in agar, gel, or sausage form. Advantages include the ability to formulate and feed a nutritionally complete diet, to add a balanced vitamin and mineral mixture, and, if needed, to add antibiotics or coccidiostats. Mostly a complete diet is fed in a sausage; however, tests are also being done with gel feeding to reptiles.
The scent of preferred foods can be rubbed on the new item. Alternatively, the preferred foods can be inserted into, or attached to, the new food. Anoles, yellow rat snakes, frogs, and smelt, depending on natural feeding habits, can be fed when homeotherms are not accepted. Prey size is usually proportional to snake size and should not be much larger in diameter than the snake’s head. Snakes that are routinely handled can be fed in a separate tank to reduce biting. To reduce the chance of regurgitation, snakes should not be handled for 3 days after feeding.
Most species should be fed every 1–2 weeks. Some large, less active snakes may typically go 6 weeks between feedings. Force-feeding should be used only if necessary. Animals can be force-fed whole prey lubricated with egg white by gently inserting the food a few inches down the throat using forceps. Tube feeding is also possible using ground (homogenized) prey.
(Divers, 2022 ![]() )IV
)IV
Diet of Reptiles The nutritional requirements of reptiles are still being investigated. Most recommendations are based on experience and observation.
Feeding behavior and digestion are related to the environmental temperature. Because reptiles have a lower metabolism than mammals and other “warm-blooded” animals, they feed less frequently. Humidity, light, food type, and the presence of other animals also affect feeding behavior. In turtles and some plant-eating lizards, the color of the food contributes to food acceptance; red and yellow are often preferred colors. Some reptiles become accustomed to certain foods and are unwilling to accept alternatives. Providing a variety of foods at each feeding, especially to younger reptiles, may lessen this problem.
Quality is important when feeding whole-animal foods. Goldfish, mealworms, crickets, wax moth larvae, mice, or rats intended for use as reptile food should be fed a complete and balanced diet so that they provide adequate nutrients. Herbivores (animals that eat plants) and omnivores (animals that eat meat and plants) also require balanced rations. Vegetarian diets are often lacking in calories, protein, and calcium. Insects and grubs lack calcium, and supplementation is required.
Gut loading is a common technique that involves giving insects a nutritious mixture of cereals and vegetables immediately before being fed to the reptile—thus loading their gut with nutrients. Another common practice is the use of powdered vitamin and mineral supplements. Crickets brought home from a pet store and never fed have little nutritional value. Placing them in a bag with vitamin and mineral powders and shaking the bag will coat the insects with the powder. Although some of the powder will fall off, the newer microfine powders adhere remarkably well. Adding calcium and calcium-rich foods to the diet of crickets and wax moth larvae is another way to provide more calcium to the reptile.
Nutrient Requirements The recommended minimum protein content of a reptile diet is about 30% to 50% for meat-eaters and about 18% to 22% for plant-eaters. Inadequate protein levels result in weight loss, muscle wasting, increased chance of infection, failure to reproduce, and slower healing after injury. An infection that will not go away after treatment can be the result of inadequate nutrition. High-protein commercial diets may prompt rapid growth but can have severe longterm consequences such as hyperuricemia (see below).
Carnivores (meat-eaters) should be fed whole animals, not just muscle meat. Feeding large amounts of high-protein cat foods has been shown to cause increased levels of protein and vitamin D3. Many nutritionists recommend not feeding cat foods to reptiles. Dog food, especially low-fat varieties, can be used sparingly as part of a complete and balanced diet in both meat eaters and plant eaters. Feeding excess protein can result in a condition called hyperuricemia, in which uric acid is deposited in internal organs. This may lead to gout of the affected organs, which can be fatal.
Protein deficiencies can occur in reptiles with poor appetites or in herbivores (plant-eaters) eating diets deficient in protein. Diets for herbivorous reptiles should include plant proteins from vegetables and legumes. Reptiles that refuse to eat may require a change in their environment or enough variety in the diet to identify a preferred food item.
Fiber is required for the normal functioning of the digestive tract. In large land tortoises and other plant-eating species, adding roughage (such as hay) to the diet may eliminate chronic, smelly diarrhea.
Specific fatty acid requirements have not been determined for reptiles. Dietary linoleic acid is recommended for some species.
Mineral deficiencies are seen frequently in captive reptiles, especially turtles, tortoises, and lizards. Vitamin and mineral deficiencies are rare in snakes that are fed nutrient-rich whole prey. A vitamin and mineral supplement should be added to the diet of every captive reptile; many products designed for use in reptiles are available in pet stores. Properly balanced calcium, phosphorus, and vitamin D3 levels are necessary to maintain good health in reptiles. If the balance is upset, hormonal disorders or bone diseases can occur.
Calcium imbalance is a common mineral imbalance in reptiles. For carnivores, a diet of only muscle meat (not whole prey) is deficient in calcium and rich in phosphorus. Including whole prey or a low-fat dog food in the diet is recommended. Calcium should be supplemented with products developed for reptiles.
The exoskeleton of insects does not contain calcium. Therefore, reptiles that feed primarily on insects must obtain dietary calcium from insects “gut loaded” and powdered with calcium supplements. Plant-eating reptiles should be encouraged to eat items rich in calcium, including cabbage, kale, okra, sprouts, collard greens, and bok choy. These foods typically are also rich in vitamin A. A calcium supplement developed for reptiles should be routinely given to plant-eaters.
Vitamin D is also required for proper calcium metabolism and balance. Animals housed outside with access to natural, unfiltered sunlight usually have adequate levels of vitamin D3. Access to ultraviolet light is strongly encouraged for reptiles that are not exposed to unfiltered sunlight ( see Lighting Requirements, above). Reptiles that are fed whole mammals (such as mice) as prey generally consume adequate levels of preformed vitamin D3. The food of reptiles that eat mostly insects should be fortified by gut loading and powdering. Plant-eating reptiles that have limited exposure to ultraviolet light should receive supplemental vitamin D3. Most reptile supplements that contain calcium also contain vitamin D3. Care should be used when providing supplements, however, because excessive levels of vitamin D3 in the diet can lead to excessive absorption and use of calcium.
Inappropriate levels of calcium, phosphorus, or vitamin D can result in an imbalance in the hormone that regulates calcium, phosphorus, and magnesium levels within the body. If these levels are not balanced, the bones may become weak and begin to bow outward. This condition is most often seen in the jaw bones. Eating becomes difficult and then impossible as the jaw bones become soft. Tube feeding is needed in extreme cases. Osteomalacia (softening of the bones), kidney stones, cloacal calculi (accumulated mineral deposits, similar to kidney stones), and rickets (which also leads to weakening of the bones) are also possible results of a diet deficient in calcium or vitamin D3. Broken bones, bone deformities, and soft or deformed shells in turtles may occur. Affected animals may develop muscle twitching or muscle contractions.
Treatment consists of correcting the balance of these minerals and giving vitamin D3, if necessary, either by exposure to an appropriate ultraviolet light source or by an injection given by a veterinarian. Your reptile's dietary history would help the veterinarian evaluate for deficiencies and determine possible courses of treatment. If a calcium supplement is used in the initial stages of treatment, it should not contain phosphorus. An excellent calcium source is calcium glubionate, given on the recommendation of a veterinarian. Other sources of dietary calcium include crushed cuttlebone, crushed oyster shells, crushed or pulverized calcium lactate, or commercially available products. In severe cases, a veterinarian can give a calcium injection before giving extra calcium by mouth.
Feeding of certain green foods that contain goiter-causing compounds, such as bok choy, broccoli, Brussels sprouts, cabbage, and soy, may cause an iodine deficiency. Signs of deficiency include a lack of normal energy and activity and an abnormal swelling (goiter) at the base of the neck where it meets the chest. The imbalance is corrected by supplementing with a balanced vitamin-mineral mixture containing iodine or iodized salt (0.5% of the diet).
Vitamin A deficiency is common in captive plant-eating turtles. Box turtles appear most at risk usually because of improper diets that do not contain enough vitamin A. Signs of vitamin A deficiency include swollen eyelids, eye discharge, chronic respiratory disease, and kidney disease. The eyes may eventually remain closed, impairing the ability of the turtle to find food. Treatment consists of short daily soaks to allow the turtle to drink and wash its eyes, application of an antibiotic ointment to the eyes, and vitamin A injections given by a veterinarian. For less severe cases, vitamin A can be supplied by adding a drop of cod liver oil to the reptile’s food twice a week. Commercial vitamin products are also available for reptiles. Dietary levels of vitamin A should be increased for up to 6 weeks before hibernation in turtles and tortoises. However, caution should be used when supplementing because too much vitamin A can cause severe thickening and irritation of the skin as well as incomplete and inadequate shedding of the skin.
Vitamin B1 deficiency can result from diets containing fish with high thiaminase levels. Giving extra vitamin B1 is required in such cases. Weight loss even though the reptile is eating enough food is a common sign, but neurologic problems (such as paralysis and lack of coordination or balance) can also occur. Some fish species contain more thiaminase than others. Frozen fish have increased thiaminase levels. Deficiencies of the water-soluble vitamins often involve more than one vitamin and require treatment with a multivitamin preparation.
Deficiencies of other vitamins and minerals sometimes occur in reptiles and can be diagnosed and treated by your veterinarian.
已下未读:【】【(Dierenfeld ![]()
![]() )IV 】【】
)IV 】【】
已下未读:【】【(Arbuckle, 2010 ![]()
![]() )IV 】【】
)IV 】【】
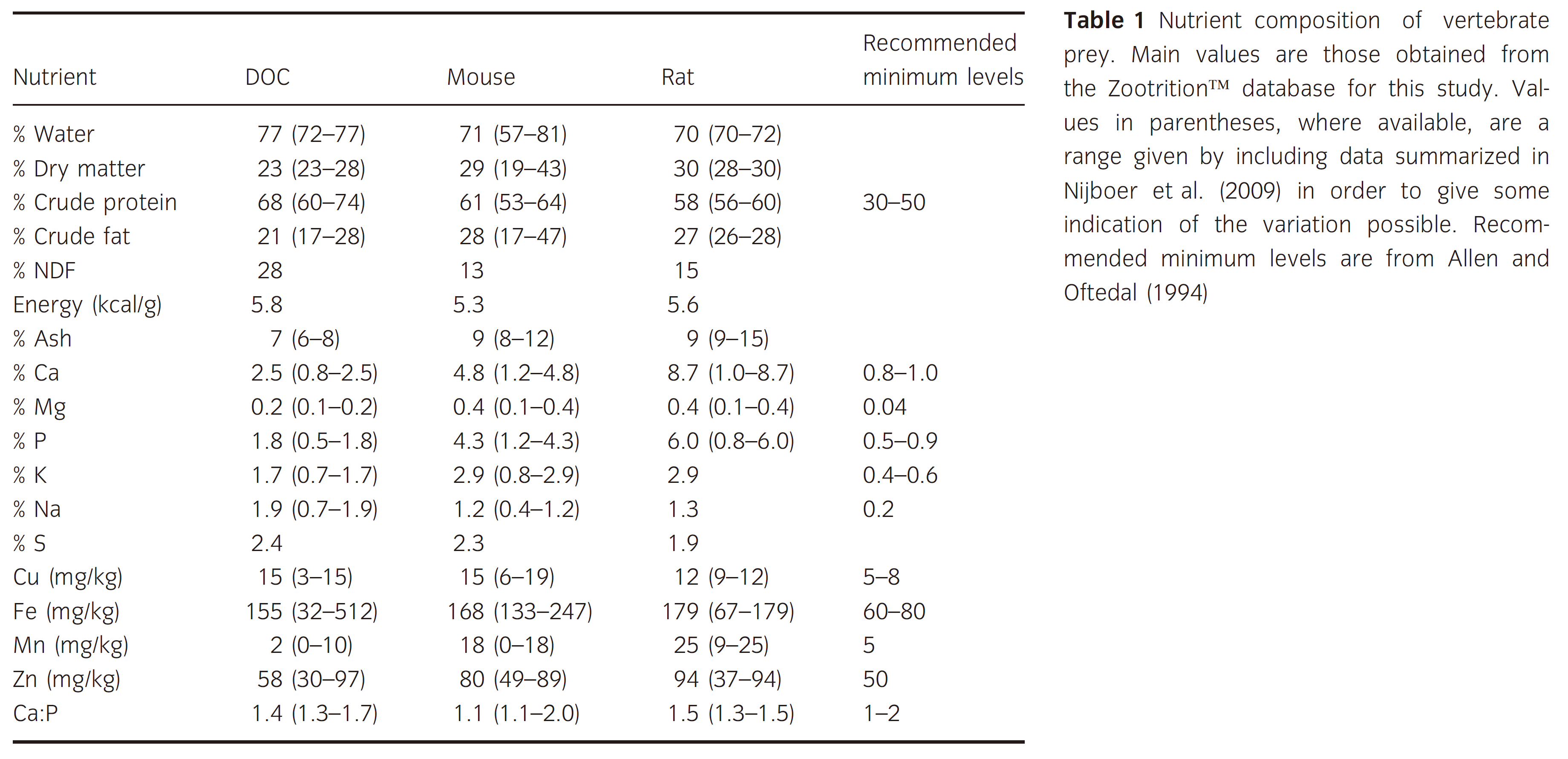
下图对比了一日龄鸡(DOC,day-old chick)的营养与小鼠大鼠的对比结果。
喂水煮蛋的讨论:
https://www.reddit.com/r/cornsnakes/comments/165mqlq/boiled_eggs/
https://www.reddit.com/r/cornsnakes/comments/169wct1/i_had_to_try_it/
https://www.youtube.com/watch?v=tTW2nuVTMJQ&lc=Ugyi0NA_XYEb5mNC_jp4AaABAg.9vlOy3h3apE9wEMVA9nVBg
Take E. coli as an example, it reproduce every 20 minutes under ideal temperature (35~40 C), it drops on both ends of the spectrum, but it drops faster on the hotter end. At 45 C, it drops to <5% of the rate. At 25 C, it drops to ~20%.
By that data, I support thawing it in hot water. If you put it in 50 C water, it would thaw within 15 minutes, and you can take it out of the bath above something like 42 C. There won't be enough time to let the bacteria grow, and most of the time the temperature is not suitable (<10% peak rate).
If you put it in 25 C room temperature water, you need more time for it to thaw (haven't tried, maybe an hour?). And it will have >20% peak reproductive rate during the time.
整体食用,需要带毛、带骨
小鼠和大鼠的区别
食物丰容
鹌鹑苗
https://www.reddit.com/r/cornsnakes/comments/15wx8ql/cornsnakes_can_regularly_eat_chicken_and_quail/
Reptilink
喂食时间表
时间表喂食计划是比较过时的,推荐使用Behavior-based feeding schedule:
https://youtube.com/playlist?list=PLNbZzsRecQ2aZi4CfONNKQOq_UUowACdZ&si=6blF0goGAun7LLrz
蜕皮时应不应该喂食(见解剖章节)
蛇是机会觅食者,总是非常饿
下文中的“冻食”指充分化冻后的冷冻食物,不是冰着喂给蛇。
长时间冷冻的食物会导致营养流失,维生素减少、且可能被冰箱内的细菌环境沾污。冷冻食物的储存最好不超过6个月(成体两周喂食一次,每次购买食物不应超过12只)。
蜥的知识:钙磷比问题,磷会剥夺身体吸收钙的机会,长期使用钙磷比较低的食物会导致缺钙
在窝里喂还是拿出来喂
在栖地里用盘子把盘子反过来的方法,如何让蛇上盘子
不要用软的材料放食物,下面是一个尝试吃掉垫子的例子:
https://www.reddit.com/r/snakes/comments/17j7mms/this_idiot_missed_the_mouse_bit_the_blanket/
(Bilibili-虚空蛋黄酱_, 2023 ![]() )I :
)I :
所谓误食垫材问题
冻食的解冻方法
冻食如何夹
食物的种类 食物大小的变化
How to tell if your snake is a proper weight
Power feeding 问题,寿命减半
生长曲线
已转录,未整理:【】【(Sinclair, 2021 ![]() )III 】【】
)III 】【】
【】【本段未整理】【】
(Sinclair, 2022 ![]() )III
)III
不要觉得眼睛能看出来就不称蛇,一定要用称称,并做好记录,及时发现问题。蛇一斤重的时候少二三十克体重肉眼是看不出来的,等到肉眼看出来的时候可能问题已经严重了。
圈养下玉米蛇的主要食物是小鼠(mice),注意不是大鼠(rat)。幼年至成年玉米蛇的食物正好对应幼年至成年的小鼠 III。
作为丰容措施,不应该让蛇一辈子只吃一种食物,可以偶尔喂食合适大小的带毛鹌鹑苗。也可以考虑偶尔喂食大鼠,但是因为大鼠的脂肪含量比小鼠高(2530% vs 1520%),(Dierenfeld ![]()
![]() )IV 所以大鼠“更好吃”,因此喂食大鼠有一定可能造成后续拒食小鼠I。
)IV 所以大鼠“更好吃”,因此喂食大鼠有一定可能造成后续拒食小鼠I。
Reptifiles表示也可以喂食鹌鹑蛋,待考察。
可以考虑喂食幼龄鸡,如一日龄鸡(Arbuckle, 2010 ![]()
![]() )IV ,注意鸡仔出生就三四十克,养一周就60克了。
)IV ,注意鸡仔出生就三四十克,养一周就60克了。
Reptilink是用动物肉搅碎后制成的香肠,对蛇来说更好吃,有时可以用于开食 III。
【】【Reptilink图】【】 【】【Snake Discovery Reptilink视频】【】
食物应该完整投喂,包括内脏、骨骼、头部、毛发或羽毛。不要去除内脏、不要褪毛。III
食物不应该脱毛,食物的毛起到膳食纤维的作用,帮助消化的排便。
排便时角蛋白组分(比如老鼠毛)会团成一团拉出来,这一团团的叫felt(毛线团)。IV
蛇可以消化几乎所有的骨骼(包括鸟类的喙、鼠的牙齿等)并从中吸收钙质。
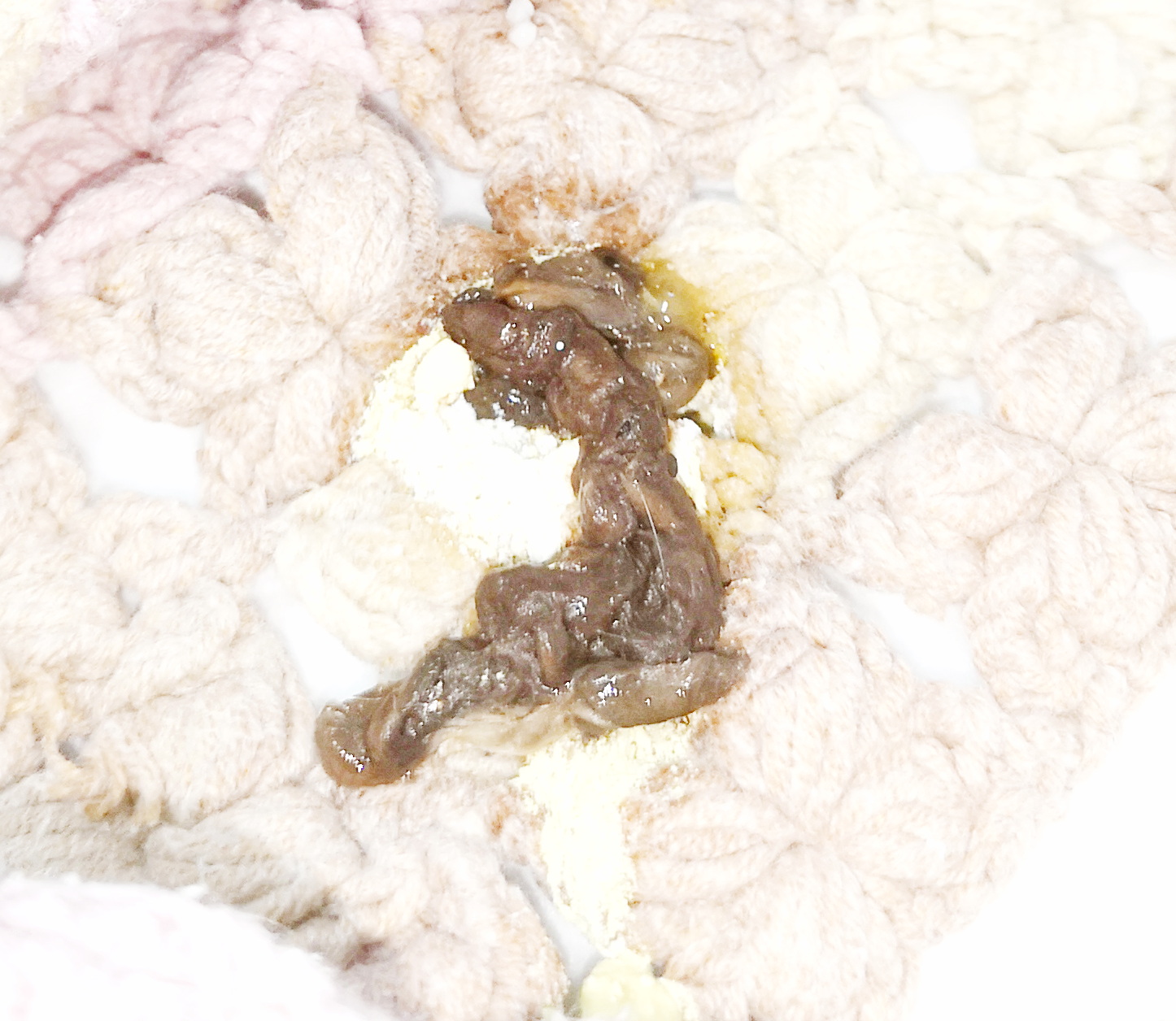
知道蛇粪便的正常状态,下图为玉米蛇的粪便,没有骨,其中黑色部分主要为老鼠毛组成的felt,浅黄色部分为尿酸。如果有骨、没felt、没尿酸、甚至还有老鼠的形状,那不是屎,是吐食了。
下图通过调亮展现了玉米蛇粪便中由小鼠的毛而形成的一团一团的felt:
应重视给玉米蛇喂食时不能褪毛。食物的毛(羽毛)是蛇的膳食纤维,帮助排便。如果长期喂食如褪毛的鹌鹑苗,会导致排便异常。
食物整只喂,不能去骨;更不能喂切块的精肉。
【】【https://www.bilibili.com/video/BV14r4y1W7Wj?spm_id_from=333.880.my_history.page.click】【】
没有找到高证据级别的喂食时间表
下列表格来自Reptifile非常存疑,来自一个Facebook社区,什么乱七八糟的? I
| 状态 | 身长(cm) | 体重 | 喂食重量 | 喂食间隔(日) |
|---|---|---|---|---|
| 幼体 | 20~50 | 5-7 | ||
| 少年 | 50-115 | 7-10 | ||
| 亚成 | 90-130 | 10-12 | ||
| 成体 | >90 | 14-21 | ||
| 老年 | 年龄>18岁 | 10-14 |
蛇超重或者过瘦的时候可以在上下限的基础上再调整1/3。例如肥胖的成体可以28天喂一次,消瘦的成体可以10天喂一次。同时可以调整食物的重量 III
来自Roy Munson,被称为“Munson plan” I,广为流传,但,一些人批评这个计划 I: 幼蛇阶段喂的非常激进(超过了20%体重,一般认为不应该超过15%) 喂的过于频繁,成体可2~3周喂一次。 根据重量的喂食计划不合理,因为在太重、太瘦的蛇身上会形成正反馈“失控”,根据身长或年龄更合理。
身长 (cm) 体重 喂食重量 喂食间隔(日)
---------- ----------- ------- ---
<25 g 25% 体重 5-6
25-50 g 20% 体重 5-6
50-90 g 15% 体重 6-7
90-170 g 10\~15% 体重 7
>170 g 10% 体重 10-14
Reptifile 喂食大小 III: 提供的猎物大小应在蛇体最宽处的1-1.5倍之间。
一般来说一顿饭不应该超过蛇体重的大约10%。这个比例要根据蛇的胖瘦调整。
对于是否适宜喂多只鼠,有争议。
Reptiles and research :Cali king wild diet implication for care 根据对加州王蛇野外食谱的大规模研究认为在野外常有一次吃很多只幼崽的情况(nest raid),也有先把妈吃了、再把窝里小的都吃了这种一大多小的情况。所以认为可以多只喂食,并且建议通过喂食大小的波动提供喂食丰容。(个人疑问:饥一顿饱一顿是自然界常态,但在人身上认为威胁健康;这一现象是否应该划入可模仿自然而不带来负面影响的范畴?)
Snake Discovery依据相同“长度”下的营养总量认为应该尽量选一只大小合适的,不要分开喂两只。
应当记录喂食的时间,食物的重量,每天+喂食前测一下体重(除了刚喂食后的48小时),记录捕食状况,是否需要辅助进食,记录拉屎的时间和拉屎后的体重,注意屎的状态,注意吐食问题。【】【吐食链接】【】 III
如果出现体重减少、或者小蛇的体重增长停滞,尽快寻找原因,很可能有病要找兽医
钙粉、维生素粉? 一般认为不需要, Reptiles and Research喂鸡的视频里有文献。 注意长期冷冻导致的营养流逝 UVB问题 小鼠、大鼠、鸡苗的钙磷比都合适
不要用手接触老鼠,否则手上有鼠味可能会被咬
【】【】【】 蛇的饲养者中有一种普遍的误解,认为在蛇的家里喂食会使蛇变得“栖地内攻击性”。现代对蛇心理的理解认为,我们对“攻击性”的认知是错误的;那些已经学会将开笼子与食物(由于处理不频繁)联系起来的蛇会向它们看到的第一个物体发起攻击,以为那是食物——并无对饲养者的手有任何伤害的意图。 III
【健康蛇、健康环境】蛇不会因为误食一般垫材而Impaction。 III
因此,不应该将蛇移到别处喂食,而应该训练蛇分辨喂食时间和处理时间。最可靠的方法是在处理前用纸巾卷轻轻敲蛇,或者用蛇钩轻轻划过其身体。如果蛇攻击,没有伤害。如果没有,它知道不要期待食物。
总之,应该在蛇的家里喂食。与人类不同,蛇并不特别喜欢“外出就餐”。
【翻转盘子栖地里喂食的方法】
【】【】【】
目标训练
【】【下面未整理】【】
https://www.youtube.com/watch?v=wd95C_abSZE Use This Snake Enrichment Idea To Encourage Hunting Behaviour
Role: assistant In the wild, snakes can spend large proportions of their time hunting and engaging in natural behaviors, anything from following the rodents entrails to rodent urine around nests sites highlighted by UVA vision. However, in captivity, the amount of time spent engaging mentally is short compared to that of the wild. In this video, we will look at how you can extend the amount of time your snake engages in these behaviors. This channel is dedicated to improving reptile welfare with science and good information sources. If you want to stay up to date with science-based care for your reptiles, click the subscribe button and press the bell icon for future information. 在野外,蛇可能会花大量的时间进行狩猎和自然行为,这些行为包括追踪啮齿动物的内脏到巢穴周围的尿液,这些都被紫外线视觉突出显示。然而,在圈养环境下,精神参与的时间相比野外要短很多。在这个视频中,我们将探讨如何延长你的蛇进行这些行为的时间。本频道致力于用科学和优质的信息来源改善爬行动物的福利。如果你想跟上基于科学的爬行动物护理的最新信息,请点击订阅按钮并按下铃铛图标以获取未来的信息。
The time an animal spends in any given day is called an activity budget, and that is the time it allocates to different behaviors on a daily basis. This strongly affects its fitness by deterring interaction rates with resources, predators, and competitors. According to this study in ethology, studies are carried out by observing different behaviors, defining them, then observing the animal for an extended period of time and recording the amount of time the animal spends doing what behavior. In captivity, the activity budget of the wild counterparts has been studied. We can utilize methods such as enrichment and enclosure design to try and encourage the animal to behave as close to that of being natural. 动物在任何给定的一天里花费的时间被称为活动预算,这是它每天分配给不同行为的时间。这通过阻止与资源、捕食者和竞争者的互动率,强烈影响其适应性。根据这项在行为学中的研究,研究是通过观察不同的行为,定义它们,然后观察动物一段较长的时间并记录动物花费在什么行为上的时间来进行的。在圈养环境下,我们已经研究了野生对应物种的活动预算。我们可以利用丰富的方法和爬箱设计来尝试鼓励动物表现得尽可能接近自然状态。
While the exact activity budget for your chosen species of snake might not be known or studied, we can safely say that they're spending more time hunting in the wild than those few moments it takes for a snake to strike at food presented in tongs. That's why it's important to try our best to extend the amount of time our animals spend hunting. During this time, they are mentally stimulated. While I have not tested this on species many considered to be sedentary ambush hunters like Gaboon vipers or blood pythons, the vast majority of colubrids and other pythons etc. will benefit from this. I have seen this used from anything from corn snakes to retics, some carpet pythons. 虽然你选择的蛇种的确切活动预算可能未知或未被研究,但我们可以肯定地说,它们在野外的狩猎时间比用钳子呈现食物时攻击的那几刻要多。这就是为什么我们要尽量延长我们的动物的狩猎时间。在这段时间里,它们得到了精神刺激。虽然我没有在很多被认为是驻足伏击猎者的物种上测试过这个,比如加布恩蝰蛇或者血蟒,但大多数的游蛇和其他蟒蛇等都会从中受益。我已经看到这个从玉米蛇到网纹蟒,一些地毯蟒等都有应用。
So what I'm doing here is placing food around the enclosure to encourage the animal to engage in those hunting behaviors. This extended the portion of the snake's activity budget to an hour and a half searching for food. To be fair, it actually carried on searching for ages after, but I only counted that from the time I put food in to the time the final pinky was consumed. It can be frustrating to watch if they really take the time of it. For that reason, I have cut sections out to make this video shorter. So what I'll do here is I'll speak sparingly so you can get the idea of how it is to watch your snake forage around the enclosure. 所以我在这里做的是在爬箱周围放食物来鼓励动物进行那些狩猎行为。这将蛇的活动预算的一部分延长到了一个半小时的寻找食物。公平地说,它实际上在之后的时间里还在寻找,但我仅从我放入食物到最后一只小鼠被消耗的时间里计算。如果他们真的花时间的话,看起来可能会令人沮丧。因此,我剪掉了部分片段以使这个视频更短。所以我在这里会少说些话,这样你就可以了解观察你的蛇在爬箱周围觅食是怎样的。
She kind of pauses here and just kind of sits under the UV for a little bit and then carries on her search. And once she found one pinky after around 40 minutes, she kind of picked it up and really took up a gear in the way she's moving around hunting. A quick wipe of the mouth and she continues with the hunt. This one was the most frustrating because it's clearly alright but yet she chooses to go the other direction. That darkened beetle was loving life though. She finally came from the other direction, up the rocks, and found the mouse pinkie and sends the darkling beetles flying into the stratosphere. Yes, oh that's a grounded hour-and-a-half and even after just finished, she's carried on cruising around the enclosure after. I didn't film that, but hopefully after watching it you've got some ideas of how you're going to increase the activity budget of hunting in your own care. Leave a comment, let me know what you're planning to do. If you do this, come back and leave a comment on the species you did it with. I would love a list of species that this has actually worked with and if someone else has the same species as you, your comment might be the thing that makes them try this. 她在这里稍微停顿一下,就在紫外线下坐了一会儿,然后继续她的搜索。大约40分钟后,她找到了一只小鼠,她捡起来,真的开始在她的狩猎方式上升级。快速擦拭嘴巴,她继续狩猎。这个是最令人沮丧的,因为一切明明都很好,但她却选择去另一个方向。那只变暗的甲壳虫却过得很快活。她最后从另一个方向来,爬上岩石,找到了小鼠,把甲壳虫送飞到了大气层。是的,哦,那是一个实实在在的一个半小时,甚至在刚刚结束后,她还继续在爬箱里巡游。我没有拍下那个,但希望在观看后你有了一些关于如何在你自己的护理中增加狩猎活动预算的想法。留下评论,让我知道你打算做什么。如果你做了这个,回来并留下你用哪种物种做的评论。我很想要一个这个实际上有效的物种列表,如果有人和你有同样的物种,你的评论可能会是他们尝试这个的原因。 Process finished with exit code 0
提供一个鹌鹑蛋
【】【喂食时的追逐捕食行为可以提高进食的满足感】【】
活食很不安全,有非常多的活食导致严重受伤案例
(Zwart, 2001 ![]() )IV 。
)IV 。
例如 (Mader, 2019 ![]() )IV :
)IV :
[IMG] 球蟒被鼠反咬撕去大量皮肤后死亡
红尾蚺被活鼠反咬导致的严重尾部损伤:
玉米蛇拒食活鼠被咬去背部并咬断脊椎导致身体残缺及瘫痪(Snake Discovery, 2024 ![]() )I :
)I :
野生的单色玫瑰蚺(Rosy Boa,Genus Lichanura,species?)被啮齿类猎物反咬后愈合造成的伤疤。(骆老师啊, 2023 ![]() )I
)I
喂食活食必须全程监看,及时干预。
(Sinclair, 2022 ![]() )III
)III
- 冻鼠易于存储。
- 冻鼠需要等半小时化冻。
- 冻鼠喂食简便,可以留在栖地内,人可以撤离,有些蛇拒食是因为怕人,将死鼠留在栖地内后能发现自主进食。
- 冻鼠喂食失败需要丢弃,不能重新冷冻。
- 活鼠必须全程监看捕食过程,及时干预。
部分蛇会拒食死鼠。但可采取很多方法使蛇开食,参见医疗-拒食章节。
应当要求卖家提供蛇可以稳定进食冻食的证明,避免自己需要解决这种问题。
玉米蛇通常有相当强烈的取食反应,因此它们对冷冻/解冻的猎物并无太大问题。
喂食活鼠可能导致未来切换回冻鼠时有困难。
鼠的饲料和繁育条件对鼠的营养价值影响很大,应当核查鼠供应商的饲养方法。参见医疗-营养、喂食、饮水问题章节
活鼠购买后自己饲养可以纠正营养问题。包括调整饮食和UVB光照。
冻存死鼠的营养价值与其生前类似,不应冷冻超过六个月。
活鼠可以作为寄生虫等其他疾病的直接转染源。鼠-蛇共患疾病较少,但仍然存在。
冷冻可以作为杀灭部分微生物、寄生虫的措施,但对某些病原体并不十分有效。参见医疗-清洁和消毒章节。
活鼠更易于发现其患病状态,但这需要鼠的医学知识,大部分人不具备。
活鼠喂食对鼠是残忍的,可能造成持续的恐惧和疼痛。相对而言,使用颈椎脱位法或二氧化碳法处死老鼠是更人道的(但其实也有各自的疼痛问题)。(Mader, 2019 ![]() )IV
)IV
直接喂食活鼠可能违反部分国家的脊椎动物福利法、或机构规定。
(如果蛇愿意捕食)寻找老鼠和捕食老鼠的过程是很好的觅食/捕食丰容措施。(Sinclair, 2022 ![]() )III , (Manrod, 2008
)III , (Manrod, 2008 ![]() )IV , (Phillips, 2011
)IV , (Phillips, 2011 ![]() )IV
)IV
如果蛇不愿意捕食,不但有咬伤风险,鼠会变成压力源,造成种间共栖压力。尤其考虑到拒食的蛇有时有医学问题,应避免。
冻食也可以实现较好的丰容效果,可通过设立气味路径、隐藏食物、增加取食难度等增加觅食丰容度。(Lori Torrini ![]() )III ,(Whittaker, 2019
)III ,(Whittaker, 2019 ![]()
![]()
![]() )IV
)IV
冻鼠购买方便。通常比较便宜、没有路损。
在大规模饲养中,自己繁育活鼠在经济上更合理。鼠的“存储”不受电力影响。
【】【Gimmel2020,玉米蛇的身体打分.pdf】【】
好视频:Lori Torrini的视频:https://www.youtube.com/watch?v=KKetLq-T-Mk&list=PLNbZzsRecQ2aD9saEbc_vO06rxDIqeSwF,有文献
【】【Snake Discovery Video】【】
https://www.reddit.com/r/cornsnakes/comments/164ywz8/underweight/:
症状 III:
- 直着的时候沿脊柱有凹陷
- 鳞片皱褶/折叠
- 蛇直着的时候能看到鳞片之间的肉(刚喂完食除外)
- 腹部是软的,不是结实
- 身体弯的时候有游泳圈,看着像分段似的
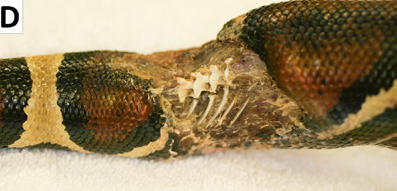
- 泄殖腔前面有明显突起(如下图),玉米蛇不应该有屁股。
单独说屁股胖是因为全身都粗可能只是生长,但只有屁股胖明显是体腔脂肪体(coelomic fat pads)里头的脂肪堆多了。
成因:
- 食材太肥,比如应该喂小鼠却喂成了大鼠(大鼠rat的脂肪远比小鼠mouse要多)
- 喂太多、太大、太频
- 蛇缺乏活动机会(如栖地太小、无丰容物、无攀爬物)
措施 III:
- 喂食正确种类的食物(比如rat换成mouse)
- 调整喂食大小和频次
- 增加丰容物、攀爬物、活动空间 【】【Snake Discovery泡澡视频】【】
- 给蛇泡澡(有特定方法,见视频),一般蛇在水里会不停游动
- 长期无法纠正时咨询兽医。
参考蛇病-消瘦与体重降低章节
【见解剖章节的对应章节】
用尿布:
https://www.reddit.com/r/cornsnakes/comments/15yakim/the_art_vs_the_artist/
【】【】【】 Live Vs Frozen for Snakes Pt 2: What Suits YOU? [pDXijDfqkJE].webm SHOCKING TRUTH About Frozen Feeder Rodents Revealed! [fyHhFvrlqiw].webm The Welfare of Rodents Used as Food for Reptiles, Huw Golledge. AHH BHS Drayton Manor Conference 2022_video.mp4" A talk by Huw Golledge, Ph.D, on the welfare of rodents used as food for reptiles at the AHH & BHS Conference at Drayton Manor on the 12th March 2022. 【】【】【】
AVMA Guidelines for the Euthanasia of Animals: 2020 Edition*
Live Vs Frozen for Snakes Pt 1: SHOCKING Truths https://www.youtube.com/watch?v=fyHhFvrlqiw
III
人道的死亡主要包括三个方面:最小化疼痛、最小化压力,并尽量缩短过程以减少痛苦。
喂活食有一定的人道主义问题,但冻食也有。
活食的人道主义问题显而易见,小鼠被捕食过程中会造成疼痛和恐惧。但这一过程相对迅速,研究表明【下方Ref】一般小鼠在40~60秒内就会死亡。不过这个数值可能随蛇的年龄、技能、认知发展、种类以及与啮齿动物的大小比例而有变化。
Brad R. Moon, The mechanics and muscular control of constriction in gopher snakes (Pituophis melanoleucus) and a king snake (Lampropeltis getula). J. Zool., Lond. (2000) 252, 83. (附件/蛇绞死老鼠的肌肉机制和速度.pdf)
二氧化碳箱处死法也有福利问题,养殖场一般用二氧化碳箱法处死老鼠。二氧化碳箱法处死老鼠通常需要2~3分钟(蛇的绞杀死亡更快是因为绞杀不仅导致窒息,还会立即导致循环和心脏停止),并且二氧化碳造成的迅速晕厥不一定是无痛的。2013年,Newcastle大学发表共识认为二氧化碳对老鼠和大鼠的厌恶程度远低于实际需要引起无意识的程度。因此,他们一致认为应寻找二氧化碳的改良方法。
Mark J. Prescott. A Good Death? Report of the Second Newcastle Meeting on Laboratory Animal Euthanasia. Animals 2016, 6, 50. (附件/二氧化碳箱法处死的人道主义问题.pdf)
University of Newcastle, Newcastle Consensus Meeting on Carbon Dioxide Euthanasia of Laboratory Animals. (附件/Newcastle大学CO2安乐死方法人道注意共识及可能的替代方案.pdf)
这些方法都比老鼠药、粘鼠板、老鼠夹等人道的多。
笔记:
饲料动物、实验动物的人道处死
(如只需查看推荐的处死方法,可跳过下面几段基础知识)
人道处死的原则:
- 尽可能无痛的使动物失去意识
- 尽可能避免动物的恐惧、挣扎、喊叫
- 尽可能缩短动物从意识到处死正在进行到完全失去意识的时间
- 方法容易操作
- 不能影响被处死动物的用途,如饲料动物不能影响食用安全、实验动物不影响所需获取的数据
处死方法分类:
- 物理
- 急性失血:切断股动脉放血,或摘除鼠的眼球放血,死亡事件约 3~5 分钟;显然会造成血液流失,导致尸体组织贫血、损失部分营养。
- 断头:用剪刀突然切断头部,切断血液循环,仅能用于恒温动物。变温脊椎动物耐缺氧能力强,不能使用此方法。脏器含血量少,适于脏器标本采集。
- 空气针冠状动脉阻塞:将大量空气注入大静脉血管(如于兔的耳缘静脉注射),导致冠状动脉阻塞,阻断血液循环;适用于较大的动物(如猫狗),兔、猫的处死需20
40 ml空气,犬需80150 ml空气。造成脏器淤血。 - (未麻醉下的)颈椎脱位:适用于小于125克的啮齿类、禽类,下方会详细介绍
- 化学
- 气体吸入:
- CO2:仅适用于小型动物,最常用,下面详细介绍
- N2/Ar:
- CO:
- 乙醚:
- 三氯甲烷:
- 注射剂:不适用于本文目的,且涉及敏感物质名称,不记录。
- 气体吸入:
一定注意人员安全 应当安装传感器
CO2法
箱内所需浓度?
血pH的快速上升会造成麻醉效果。吸入7.5%的CO2可减少疼痛,吸入30%的CO2可以快速导致深度麻醉
方法有直接把动物放到CO2箱中,也有gradual displacement
CO2造成压力有三个途径:1. 碳酸疼痛,2. 缺氧感,3. 直接通过离子通道刺激amygdala(扁桃腺?)导致恐惧反应。这些压力因素有巨大的物种差异。
人、大鼠、猫对CO2的伤害响应于CO2>40%时触发,人汇报难受是3050% CO2,超过50%有明显疼痛。大鼠中使用100%的CO2会触发nociceptor(导致负面体验?),但1050%不会。这说明gradual displacement可能是更好的方法。
箱内CO2释放出后的屋内CO2浓度是否危险?
气源可以使用蚁人动力的气阀,配3/8-24UNF螺口的气瓶
箱子的尺寸和所需CO2的量?
确定负面体验的方法是平行箱实验(aversion experiment),即设置两个条件相似的箱子,使鸟自由选择进入哪一个。如果鸟避免了其中一个,说明该箱会导致负面感受。
(AVMA guidelines for the euthanasia of animals) 对火鸡来说,发现会自愿走入90% Ar,或60% Ar + 30% CO2的箱子中,但只有50%走入了70% CO2的箱子中。
超过40%浓度的CO2气氛可以同时麻醉并随后杀死动物。
可能出现无意识挣扎情况
未孵化鸟类的环境通常有高浓度二氧化碳,所以刚孵化鸟类的二氧化碳耐受能力高。此时需要80%~90%的CO2
施加CO2时,有的文献报道有行为stress,但有的没有,并不是都能重复,说明可能与施加方法有关。
鸟类比鼠类更愿意接受可以导致失去肢体控制能力的高浓度CO2环境,并很快就失去意识。
7日龄鸡在97% CO2下于12s内失去意识,一日龄鸡在90% CO2下12秒内失去意识。5周龄鸡在60% CO2下17秒内失去意识。
使用gradual displacement的方法,动物在被高浓度CO2导致疼痛之前就失去了意识,所以是更温和的方法。对禽类gradual displacement of CO2可以比N2和Ar有更少的convulsion.
CO2可能导致无意识挣扎,如扑扇翅膀。可能导致观察样本损坏,让操作者难受。CO2导致的无意识挣扎比N2、Ar少。更慢的施加气体可以减少这种现象。
注意CO2比空气重,所以处理不当时容器上方空气较多,必须完全的排出空气,否则动物可能通过伸长脖子呼吸上方空气导致处死失败。
干冰的购买比二氧化碳气瓶方便,有人将干冰放于杯中,加入水加速升华,然后将此杯与小鼠一起放入较高容器中,使用升华出的气态二氧化碳处死小鼠,但目前不推荐直接用干冰直接做CO2源,其低温和水雾是额外的压力因素,应该直接使用常温气态二氧化碳。
对鼠类,建议使用0.3~0.7 倍容器体积/min的速度做gradural displacement
CO2气流应该在呼吸停止后继续保持一分钟。
幼年动物必须长时间保持在CO2气氛中保证死亡。幼年动物耐受CO2能力强,达到快速麻醉的浓度可能高达80~90%。对一日龄鸡,要保证致死,需要在>75%浓度下保持5分钟。
不能将动物突然置于100%CO2中,这不人道。如果不能用gradual displacement,可以先使动物失去意识,再放入100%CO2箱中。将禽类放在少一些浓度的CO2箱中是可行的(少多少啊?Immersion of poultry in lesser concentrations is acceptable with conditions as it does not appear to be distressing.)。
应当确认死亡,避免CO2撤除后“复活”
惰性气体法(如Ar或N2) 研究发现100%的Ar或100%的N2环境可以快速杀死动物(11s昏厥,22s停止抽搐,1min内无心电),十秒内施用惰性气体再快速换为空气时鸟类无明显反应,说明鸟类没有N2和Ar的传感器。
大鼠可以感知氧气浓度的变化,在平行箱实验中会避免纯N2, Ar环境;N2, Ar比CO2好一点,但区别不大。在gradual displacement过程中,大鼠会在O2 ~7%时主动逃离环境,几乎不会无意识的昏迷。
但鸟类(鸡、火鸡)就没有上述问题。会主动进入90% Ar的房间
认为用于鸡的处死是可接受的
注意惰性气体没有对应传感器,必须注意同屋人员的安全问题
即使是惰性气体,gradual displacement也造成aversion
要点:
此处“试剂”指的是CO2, N2, Ar等窒息试剂
-
昏迷时间与吸入试剂的浓度有很大关系。所以必须了解向密闭空间进行气体输送的规律。
-
提前让动物吸入试剂可能加快处死时间,但这本身就造成压力,所以还不如gradual displacement也造成aversion
-
加入试剂必须纯,比如不能用点火生成的CO2
-
快速加入气体可能有对应的声音或冷风,这会造成压力和逃跑行为。应当使用措施减小噪音,并避免气流直接吹向动物。
-
应该采取动物喜欢的环境进行处死,比如老鼠应该处死于黑暗的房间里,猪应该集体处死。
-
必须确认动物死亡再撤出处死环境。如果动物没有死,必须立即继续处死,否则第一次未成功处死带来的生理伤害(如肺和上呼吸道损伤)可能产生痛苦。
鹌鹑方案
处死盒,圆筒状气密饭盒,1500 ml,盖子打孔两个:第一个孔用热熔胶装4 mm导气管,长度伸至罐子底部,绑上一次性筷子,第二个是小孔,用小号钉子扎小洞
导气管接流速计出口,满量程400 ml/min
导气管入口接CO2一次性气弹控压阀,8 mm咀口;控压阀接气弹,气弹接口3/8-24UNF,
gradual displacement with CO2,每分钟0.3倍体积,即每分钟450 ml,1~2分钟达到高浓度。维持CO2 5分钟。
一盒可以放六只鹌鹑,不做上下分割。
鹌鹑在盒中会感到紧张,应该尽快转移处置
以100 ml/min流速触底加CO2时,约2~3分钟开始出现呼吸异常状态,随后一分钟内不再作动,贴住CO2放气孔,维持较小气流,保持7分钟。7分钟后取出鹌鹑,对应已死亡的鹌鹑用颈椎脱位法进一步确保死亡。
每盒体积等于3 g CO2
房屋尺寸306370250 = 28立方米,每盒CO2放到空气中对卧室来说会导致房间CO2上升28 ppm
若失误将整瓶放出,上升203 ppm,仍然安全。不要买更大的气瓶。
https://www.youtube.com/watch?v=GBU8rGGU2V8&pp=ygUfQ2VydmljYWwgZGlzbG9jYXRpb24gIGV1dGhhbnNpYQ%3D%3D
颈椎脱位术(Cervical Dislocation)
其本质是使脖子呈过度后仰角。右手拇指从脑后固定头顶,食指和中指自然夹住脖子;左手握住身体(固定好翅、腿,手势如同隔着塑料袋捡起一条狗屎),突然用力使头后仰并拉伸至极限角度和长度,不歪头、不扭头;先后仰、再低头、再后仰,并重复数次以保证死亡。
颈椎脱位时将能听到明确的声音和瘆人的手感;其声音和手感都类似于用烤鸡皮包住一根(不是一包)弯曲的干方便面。然后一弯鸡皮,干方便面碎了。
https://www.youtube.com/watch?v=vWsGc0xtiLY&pp=ygUfQ2VydmljYWwgZGlzbG9jYXRpb24gIGV1dGhhbnNpYQ%3D%3D
颈椎脱位后会有挣扎,但属于死后挣扎。如果已用CO2正确处死,不会有任何动作。
https://www.youtube.com/watch?v=VQjfIjIBaQ0
对小的动物,如小鼠,可找一隔板使其抓住板子,左手用螺丝刀等物卡住脖子(设想其是菜刀切头、但刀钝切不下去的感觉),右手抓住尾巴基部或下半身,用力向上、向头拉拽,使脖子呈后仰90度并拉长。
https://www.youtube.com/watch?v=opwXJ7MgjPE&pp=ygUfQ2VydmljYWwgZGlzbG9jYXRpb24gIGV1dGhhbnNpYQ%3D%3D
处死后先称重,清洗口腔(水冲),肛门(一定要像挤最后的牙膏一样从肛门前侧腹面把残余的屎挤出来,几乎每只都有,水冲,肛门不容易观察到的话可以浸湿肛周羽毛再观察),然后充分浸湿体表,使其亲水,以方便清洗体表,封袋速冻。
鹌鹑的饲养:
保温:
Youtube: 第一周95F=35C,第一周之后每三天降低5F = 2.5 C
卖家:1-5天36度,6-10天34.5度,11-15天32.5度,16-20天31.5度
开始时每平方小于95只,养到20天每平方小于65只
提供防溺水下水水盆。不要使用一般的碗,打湿羽毛可能影响健康。折衷方案是一个碗里放上鹅卵石,直至石头露出表面。一定注意换水,有时在水嘴里拉屎,会影响其他鹌鹑的卫生
提供饲料要够细,不吃饲料的话用手戳戳作为示范(不需要)。饲料用一次性盘子承接,鹌鹑不大时需要撕出一个缺口
箱子0.6 m * 0.35 m,养22只
会在食盘里拉屎,及时更换
第一天来的时候寄了32只,死亡7只;第二天死亡两只,第三天死亡一只,第二天体重13克;第七天有一只垂危,主动处死;另在第七天处死18只冷冻;其余三只继续养;第七天体重24~34克。
铺尿垫,撒上玉米芯,尿垫和玉米芯可能要一天清两次,屎特别多;实在来不及的话撒上新的玉米芯隔开
每日清理垫材;嫌多可以考虑隔一日清理一次,但是要掺入新的垫材并搅拌,保证干燥。
水盆放在热灯下面,保证水温